Synthesis method of 3-(2-cyanovinyl)indole derivative
A technology of indole derivatives and cyanovinyl, applied in the direction of organic chemistry, can solve the problems of high toxicity and high price of diethyl phosphate compounds, and achieve the effects of low toxicity, simple operation, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
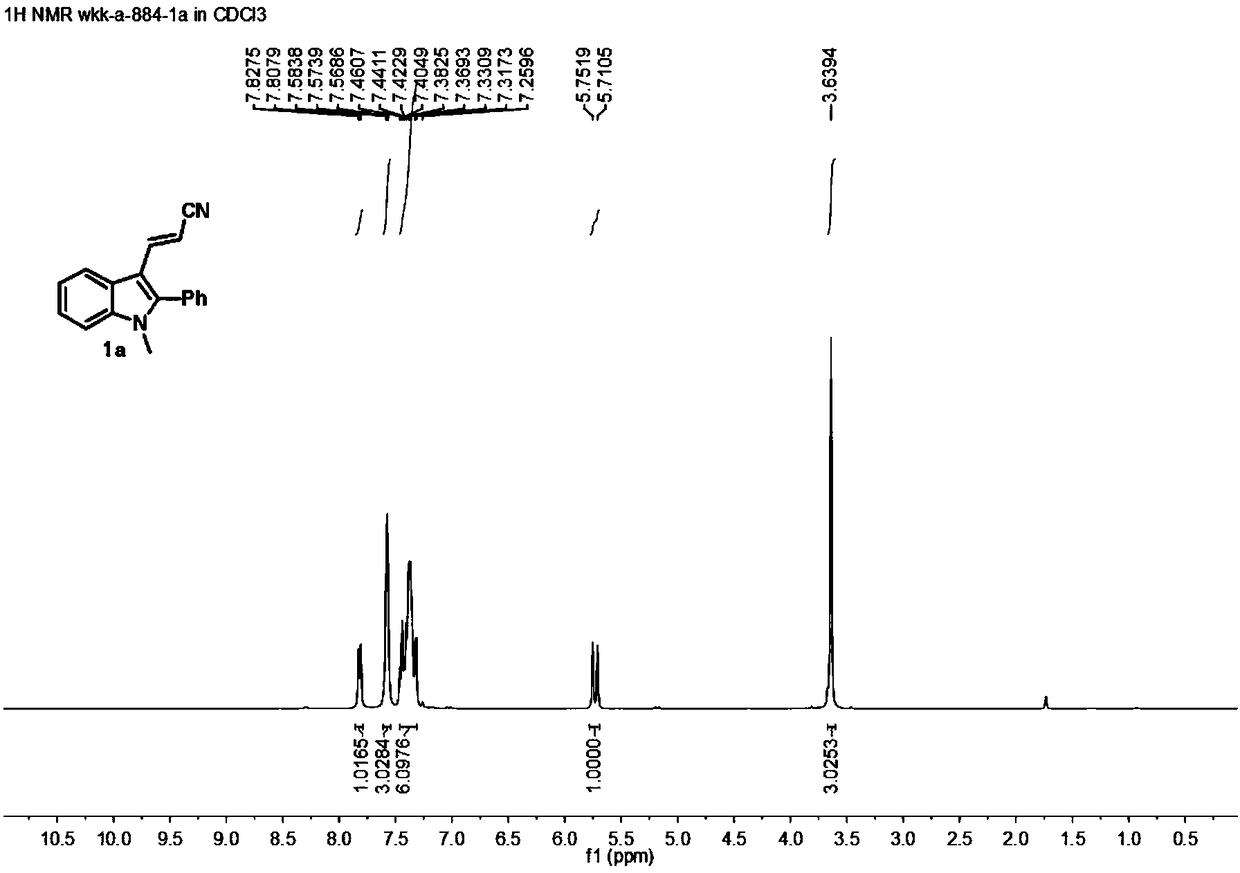
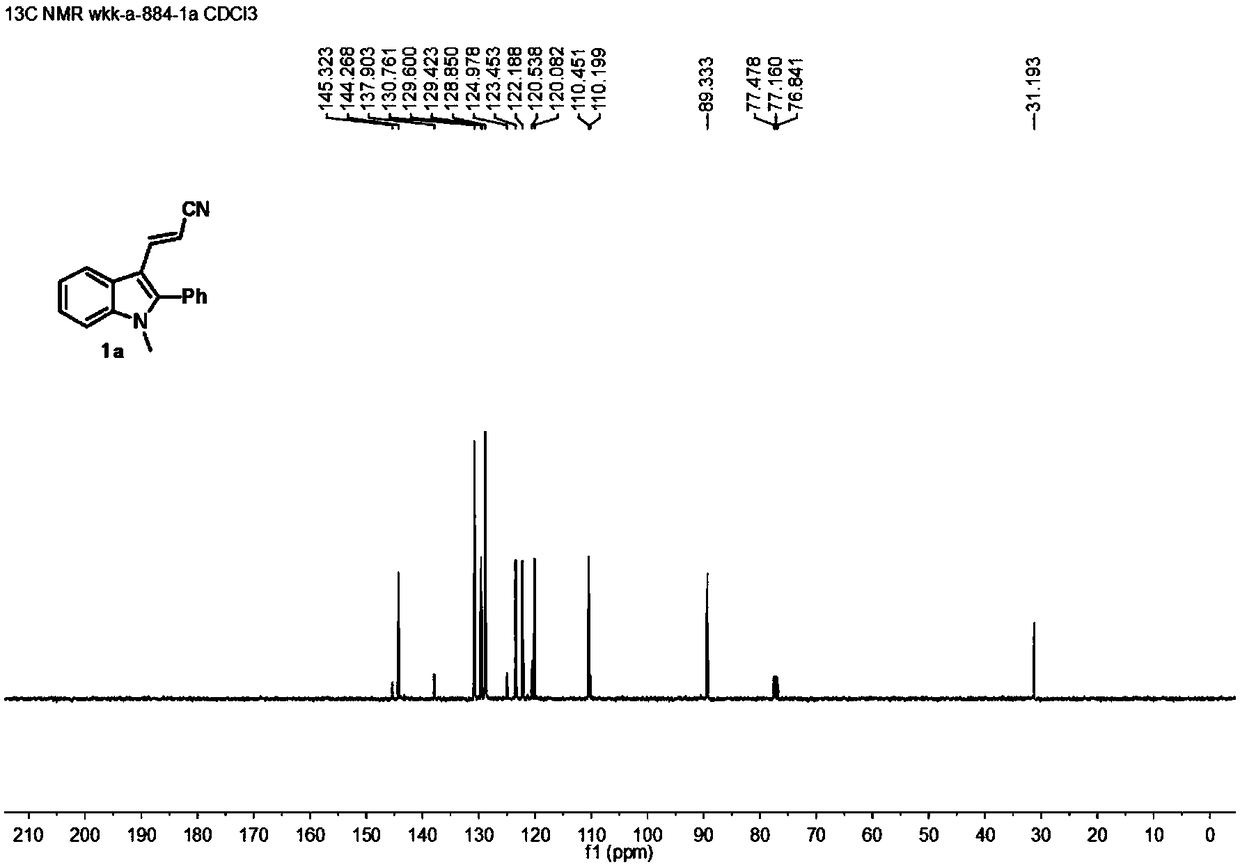
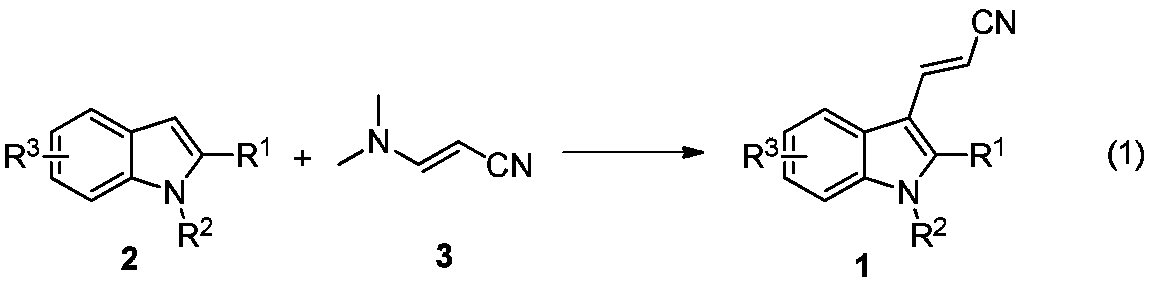
[0032] In a 10mL reaction flask, add 1-methyl-2-phenylindole (2a) (104mg, 0.5mmol), 3-dimethylaminoacrylonitrile (3) (48mg, 0.5mmol), p-toluenesulfonate Acid monohydrate (190mg, 1.0mmol), glacial acetic acid (600mg, 10.0mmol) and 2mL solvent dichloroethane were stirred at 80°C for 8h. After the reaction is completed, the reaction solution is poured into a separatory funnel, and 10 mL of saturated aqueous sodium bicarbonate solution is added thereto, shaken, left to stand, and the oil-water phase is separated, and the water phase is extracted with dichloromethane (2 × 5 mL), and the organic Mutually. The organic phases were mixed, dried over anhydrous sodium sulfate, and filtered. The volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (dichloromethane as the eluent) to obtain the target product (1a) (90 mg, yield 70%) as a yellow solid. The target product was confirmed by NMR and high-resolution mass spe...
Embodiment 2
[0034] The reaction steps and operations are the same as in Example 1, except that the reaction time is 24 hours. The reaction was stopped, and the target product 1a (89 mg, yield 69%) was obtained after post-processing.
Embodiment 3
[0036] The reaction steps and operations are the same as in Example 1, except that the reaction time is 4 hours. The reaction was stopped, and the target product 1a (44 mg, yield 34%) was obtained after post-processing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com