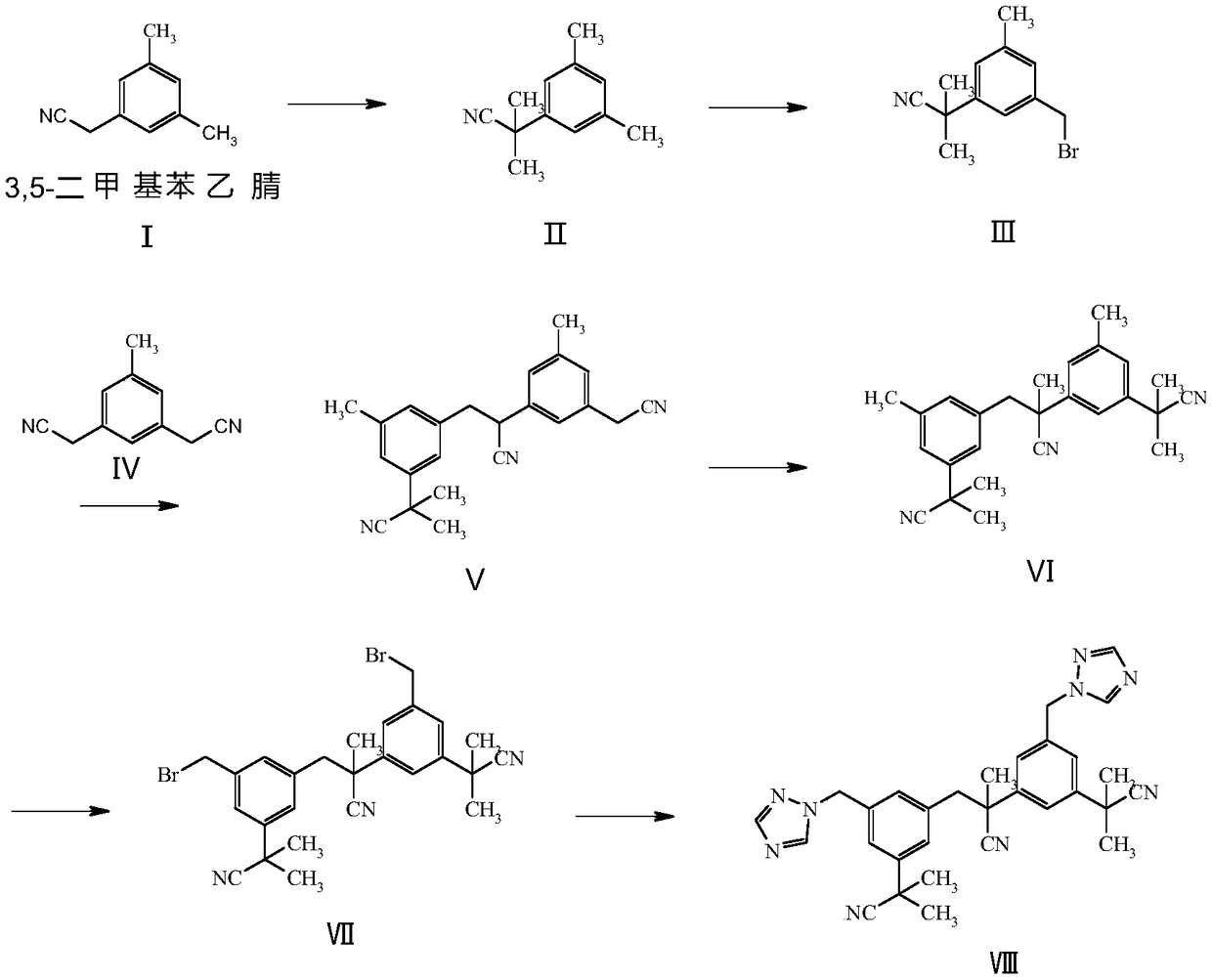
Preparation method of anastrozole derivatives
A technology of anastrozole and its derivatives, which is applied in the field of drug synthesis, can solve problems such as unreported synthesis methods of anastrozole derivatives, and achieve the effects of good environmental protection, simple operation methods, and important application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
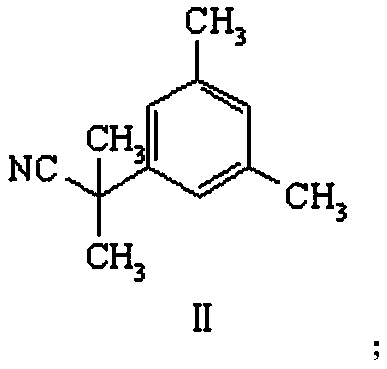
[0030] Preparation of compound II: Dissolve 0.01mol 3,5-dimethylbenzeneacetonitrile in dry 20mL tetrahydrofuran, add alkali at -50°C, stir at -50°C for 0.5 hours, the reaction solution changes from a white solution Light yellow solution, add methyl iodide, and react at a certain temperature for 3 hours. Into the reaction solution, 30 mL of saturated ammonium chloride aqueous solution was added dropwise, and extracted twice with 30 mL of ethyl acetate. After the organic phases were combined, the organic phases were dried and evaporated to dryness to obtain a yellow oily compound II.
[0031] The type of base and the experimental data of reaction temperature are as follows:
[0032]
[0033] The results of the above screening experiments show that the best base for this step is lithium diisopropylamide, and the best reaction temperature is 10°C.
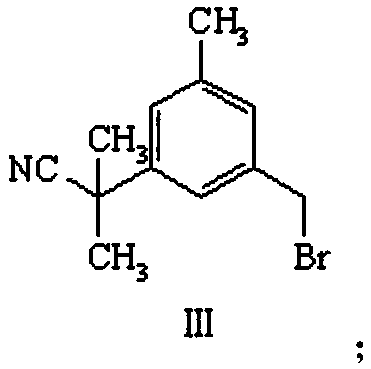
[0034] Preparation of compound III: Dissolve 0.009mol of compound II in 9mL of carbon tetrachloride, add 0.009mol of N-bromosuccinimide, ad...
Embodiment 2
[0061] Example 2 A preparation method of anastrozole derivative, which includes the following steps: figure 1 As shown,
[0062] Preparation of compound II: Dissolve 0.01mol 3,5-dimethylbenzeneacetonitrile in dry 20mL tetrahydrofuran, add alkali lithium diisopropylamide at -50°C, and stir at -50°C for 0.5 hours. The solution changed from a white solution to a light yellow solution, and then added methyl iodide, and reacted at 10°C for 3 hours. Into the reaction solution, 30 mL of saturated aqueous ammonium chloride was added dropwise, and extracted twice with 30 mL of ethyl acetate. After the organic phases were combined, the organic phases were dried and evaporated to dryness to obtain yellow oily compound II with a yield of 91.1%. The MS data of compound II is: 174.1[M+1], H 1 -NMR showed 3 hydrogen chemical shifts (6.94 to 7.07) on the benzene ring, and more than 1.7 to 2.4 methyl hydrogen chemical shifts.
[0063]
[0064] Preparation of compound III: Dissolve 0.009 mol of co...
Embodiment 3
[0074] Example 3 Functional Experiment of Anastrozole Derivatives
[0075] The pharmacological activity of the compound VIII anastrozole derivative prepared in Example 2 of the present invention was investigated, and the experimental results showed that the compound VIII anastrozole derivative can reduce the level of estrogen. Clinical observation of 120 postmenopausal breast cancer patients with progesterone receptor positive. Anastrozole derivatives have an effective rate of 42% in tumor shrinkage, and the effective rate of conversion to breast-conserving surgery for menopausal breast cancer patients who require mastectomy 36%, of which gynecological and vascular events in adverse reactions were reduced by 32%, and the recurrence rate was reduced by 35%, showing a good effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com