A kind of mirtazapine synthesis and aftertreatment method
A technology of mirtazapine and mirtazapine, which is applied in the field of mirtazapine synthesis and post-treatment, can solve problems affecting yield, low efficiency, and environmental protection, and achieve the effect of improving production efficiency and economic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
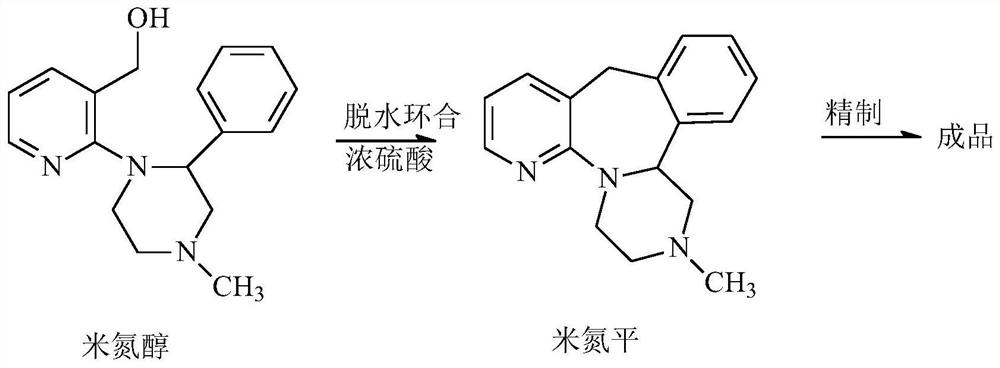
[0030] Synthesis and aftertreatment of embodiment 1 mirtazapine, comprising:
[0031] (1) Put 100g of mirazepine into 200ml of concentrated sulfuric acid in batches, control the temperature not to exceed 35°C, and then stir at 25°C for 13h;
[0032] (2) under stirring condition, drop above-mentioned concentrated sulfuric acid reaction solution in the mixture of 700g ice and 1100ml methyl tert-butyl ether, and keep reflux condensation;
[0033] (3) Add 1500g of 25% sodium hydroxide to make the pH of the aqueous solution layer be 12, heat up to reflux, stir for 5min and separate the organic layer;
[0034] (4) Evaporate the above mixed solution under reduced pressure to remove the methyl tert-butyl ether solvent to obtain the crude mirtazapine.
Embodiment 2
[0035] Synthesis and aftertreatment of embodiment 2 mirtazapine, comprising:
[0036] (1) Put 100g of mirazepine into 200ml of concentrated sulfuric acid in batches, control the temperature not to exceed 35°C, and then stir at 30°C for 10h;
[0037] (2) under stirring condition, drop above-mentioned concentrated sulfuric acid reaction solution in the mixture of 800g ice and 1200ml methyl tert-butyl ether, and keep reflux condensation;
[0038] (3) Add 1500g of 25% sodium hydroxide to make the pH of the aqueous solution layer be 11, keep reflux and condense, and separate the organic layer after stirring for 5min;
[0039] (4) Evaporate the above mixed solution under reduced pressure to remove the methyl tert-butyl ether solvent to obtain the crude mirtazapine.
Embodiment 3
[0040] Synthesis and aftertreatment of embodiment 3 mirtazapine, comprising:
[0041] (1) Put 100g of mirazepine into 200ml of concentrated sulfuric acid in batches, control the temperature not to exceed 35°C, and then stir at 20-30°C for 13h;
[0042] (2) under stirring condition, drop above-mentioned concentrated sulfuric acid reaction solution in the mixture of 900g ice and 1000ml methyl tert-butyl ether, and keep reflux condensation;
[0043] (3) Add 1500g of 25% sodium hydroxide to make the pH of the aqueous solution greater than 11, and keep reflux to condense, stir for 5min and separate the organic layer;
[0044] (4) Evaporate the above mixed solution under reduced pressure to remove the methyl tert-butyl ether solvent to obtain the crude mirtazapine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com