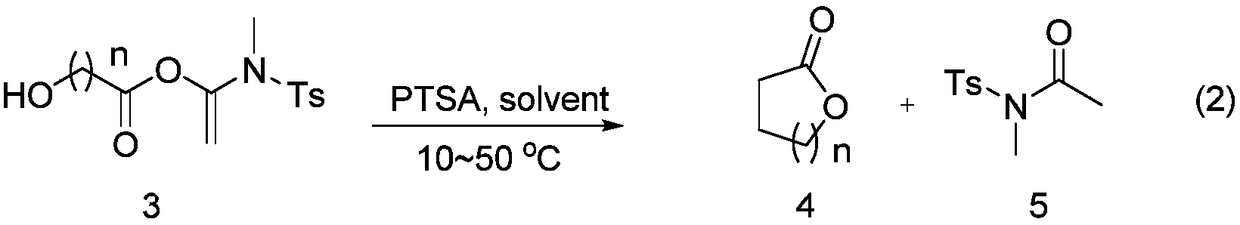
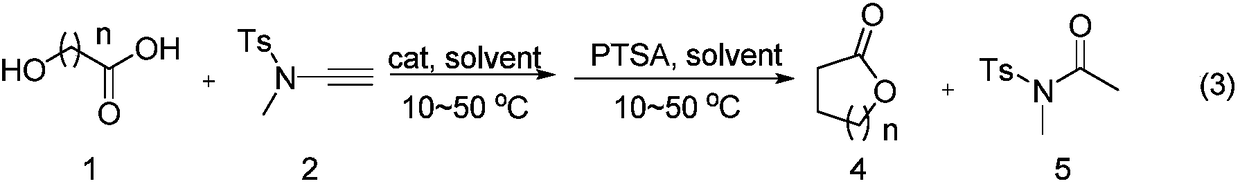
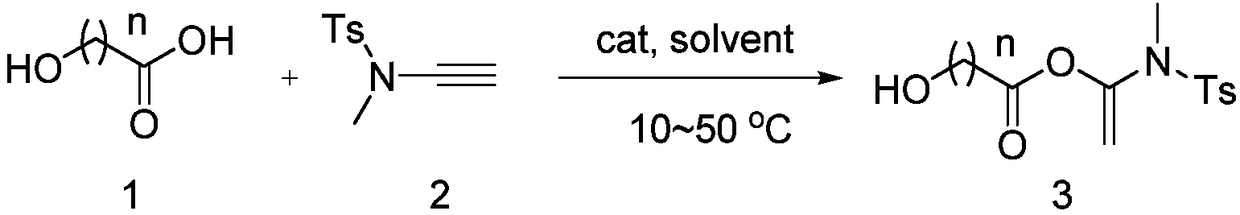Alkyne amide-mediated one-pot method for preparing macrolide
A technology of macrocyclic lactone and alkyne amide, which is applied to the preparation of sulfonic acid amide, organic chemical methods, organic chemistry, etc., to achieve the effect of realizing application value and direct and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037]
[0038] In a clean 4mL reaction flask, add alkyne amide (0.22mmol), 7-hydroxyheptanoic acid (0.2mmol), add catalyst 10mmol% CuCl, add 2mL CH 2 Cl 2 It was used as a solvent and reacted at room temperature for 24 hours, followed by TLC plate detection. After the reaction, the solvent was concentrated and column chromatography was used to obtain a pure product, a white solid, with a yield of 92%.
[0039] 1 H NMR (400MHz, CDCl 3 )δ7.7(dd,2H),7.3(dd,J=12.7,7.0Hz,2H),4.8(d,1H),4.6(d,1H),3.6(t,2H),3.0(s,3H ),2.4(s,3H),2.3(t,2H),1.7–1.5(m,4H),1.4–1.3(m,4H).
[0040] 13 C NMR (101MHz, CDCl 3 )δ171.1, 147.1, 144.1, 133.9, 129.5, 128.0, 100.3, 62.7, 37.4, 33.8, 32.5, 28.7, 25.3, 24.4, 21.5.
[0041] HRMS m / z(ESI)calculated for C 17 h 25 NaNO 5 S+(M+) + :356.1346, found: 378.1342
example 2
[0043]
[0044] In a clean 4mL reaction tube, add alkyne amide (0.22mmol), 12-hydroxyalkanoic acid (0.2mmol), add 10mol% CuI, add 2mL CH 2 Cl 2 It was used as a solvent and reacted at room temperature for 24 hours, followed by TLC plate detection. After the reaction, the solvent was concentrated and column chromatography was used to obtain a pure product, a white solid, with a yield of 93%.
[0045] 1 H NMR (400MHz, CDCl 3 )δ7.72(dd, J=8.1Hz, 2H), 7.32(dd, J=8.0Hz, 2H), 4.81(s, 1H), 4.62(s, 1H), 3.63(t, J=6.6Hz, 2H), 3.01(s, 3H), 2.44(s, 3H), 2.31(t, 2H), 1.65–1.11(m, 20H).
[0046] 13 C NMR (101MHz, CDCl 3 )δ171.3, 147.0, 144.0, 133.9, 129.5, 128.0, 100.5, 63.0, 37.3, 33.9, 32.8, 29.5, 29.5, 29.4, 29.4, 29.2, 29.0, 25.7, 24.5, 21.6.
[0047] HRMS m / z(ESI)calculated for C 22 h 35 NNaO 5 S+(M+) + :448.2128, found: 448.2127
example 3
[0049]
[0050] In a clean 4mL reaction tube, add alkyne amide (0.20mmol), 12-hydroxyalkanoic acid (0.2mmol), add 10mol% CuCl, add 2mL CH 2 Cl 2 It was used as a solvent and reacted at room temperature for 24 hours, followed by TLC plate detection. After the reaction was completed, the solvent was concentrated and column chromatography was used to obtain a pure product, a white solid, with a yield of 91%.
[0051] 1 H NMR (400MHz, CDCl 3 )δ7.72(s, 2H), 7.31(dd, 2H), 4.81(d, J=2.4Hz, 1H), 4.63(d, J=2.3Hz, 1H), 3.63(t, 2H), 3.01( s,3H),2.44(s,3H),2.31(t,2H),1.70–1.03(m,24H).
[0052] 13 C NMR (101MHz, CDCl 3 )δ 171.3, 147.0 144.0, 134.0, 129.0, 128.0, 100.5, 63.0, 37.3, 34.0, 32.8, 29.6, 29.6, 29.4, 29.4, 29.2, 29.0, 25.7, 24.5, 21.6.
[0053] HRMS m / z(ESI)calculated for C25H41NNaO5S+(M+) + :490.2598,found:490.2591
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com