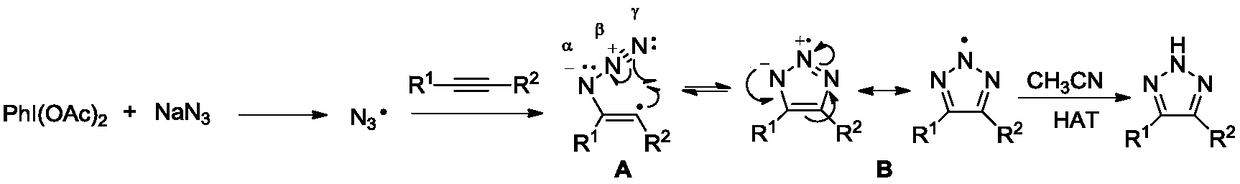
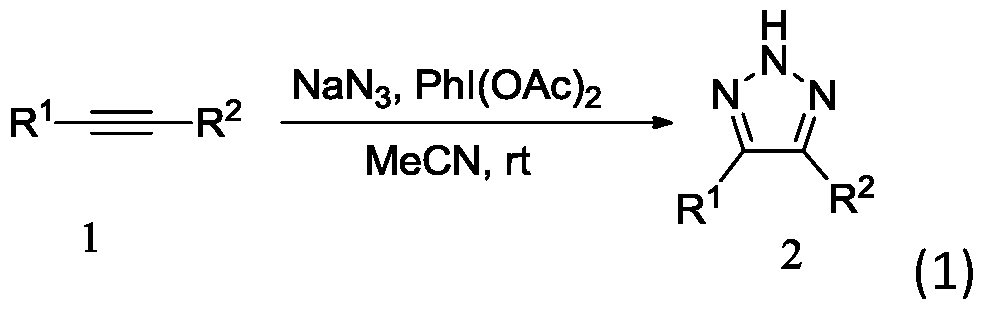A method for efficiently preparing nh-1,2,3 triazole compounds
A triazole compound and compound technology, applied in the field of efficient preparation of NH-1,2,3 triazole compounds, to achieve the effect of realizing application value and direct and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021]
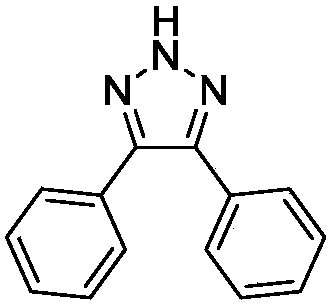
[0022] In a clean 25 mL Schlenk tube, add 1,2-diphenylacetylene (0.20 mmol), NaN 3 (0.3mmol), PhI(OAc) 2 (0.2mmol), add 3mL CH 3 CN was used as a solvent, reacted at room temperature for 8 hours, and was detected by TLC spotting. 2 SO 4 After drying, the organic layer was concentrated and subjected to column chromatography to obtain pure 4,5-diphenyl-2H-1,2,3-triazole as a light yellow solid with a yield of 95%.
[0023] 1 H NMR (400MHz, CDCl 3 )δ7.64-7.48(m,4H),7.40-7.27(m,6H).
[0024] 13 C NMR (100MHz, CDCl 3 )δ142.1, 129.9, 128.6, 128.5, 128.2ppm.
[0025] HRMS (ESI) calcd for C 14 h 12 N 3 (M+H) + :222.1031, found 222.1028.
example 2
[0027]
[0028] Into a clean 25 mL Schlenk tube was added 1-methyl-4-(phenylethynyl)benzene (0.20 mmol), NaN 3 (0.3mmol), PhI(OAc) 2 (0.2mmol), add 3mL CH 3 CN was used as a solvent, reacted at room temperature for 8 hours, and was detected by TLC spotting. 2 SO 4 After drying, the organic layer was concentrated and subjected to column chromatography to obtain pure 4-phenyl-5-(p-tolyl)-2H-1,2,3-triazole as a light yellow solid with a yield of 92%.
[0029] 1 H NMR (400MHz, CDCl 3)δ7.71–7.52(m,2H),7.44(d,J=8.0Hz,2H),7.41–7.31(m,3H),7.18(d,J=7.8Hz,2H),2.38(s,3H ).
[0030] 13 C NMR (100MHz, CDCl 3 )δ142.1, 141.9, 138.5, 130.2, 129.3, 128.6, 128.4, 128.2, 128.1, 126.9, 21.3ppm.
[0031] HRMS (ESI) calcd for C 15 h 14 N 3 (M+H) + :236.1188, found 236.1182.
example 3
[0033]
[0034] Into a clean 25 mL Schlenk tube was added 1-methoxy-4-(phenylethynyl)benzene (0.20 mmol), NaN 3 (0.3mmol), PhI(OAc) 2 (0.2mmol), add 3mL CH 3 CN was used as a solvent, reacted at room temperature for 8 hours, and was detected by TLC spotting. 2 SO 4 After drying, the organic layer was concentrated and subjected to column chromatography to obtain pure 4-(4-methoxyphenyl)-5-phenyl-2H-1,2,3-triazole, light yellow solid, yield 79% .
[0035] 1 H NMR (400MHz, CDCl 3 )δ7.60-7.53(m,2H),7.47(d,J=8.6Hz,2H),7.40-7.31(m,3H),6.89(d,J=8.7Hz,2H),3.83(s,3H ).
[0036] 13 C NMR (100MHz, CDCl 3 )δ159.8, 142.1, 141.8, 130.3, 129.5, 128.6, 128.4, 128.1, 122.2, 114.1, 55.2ppm.
[0037] HRMS (ESI) calcd for C 15 h 14 N 3 O(M+H) + :252.1137, found 252.1130.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com