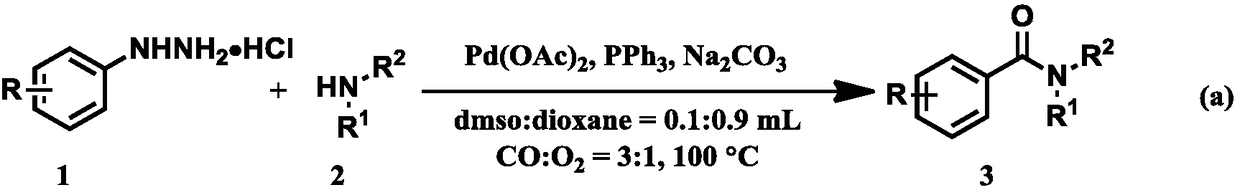
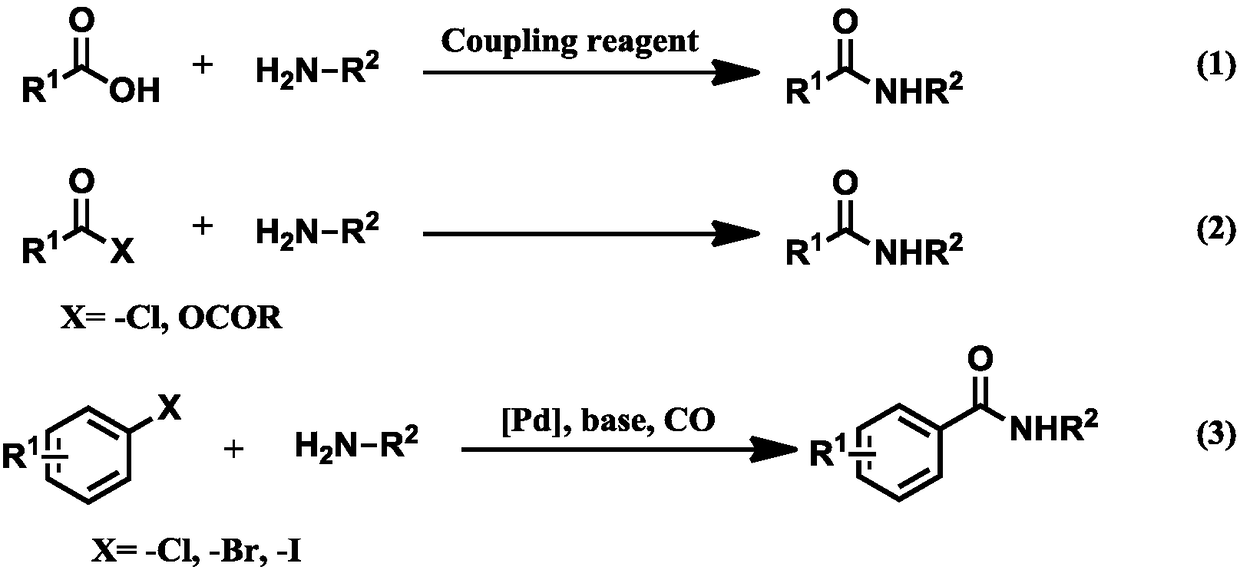
A green and environmentally friendly method for synthesizing amide bonds
A technology for synthesizing amides and secondary amines, which is applied in the field of green and environmentally friendly synthesis of amide bonds, can solve problems such as waste and excessive use of stoichiometric amounts, and achieve the effect of realizing application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019]
[0020] 1) Add 0.4mmol of phenylhydrazine hydrochloride and 0.03mmol of Pd(OAc) to a clean 25mL Schlenk tube 2 , 0.2mmol PPh 3 , 0.2mmol of Na 2 CO 3 , In CO:O 2 =3:1 in an atmosphere of 0.2 mmol of morpholine and a mixed solvent (dimethyl sulfoxide: dioxane = 0.1: 0.9 mL), react at 100° C. for 12 h, and TLC spot plate detection.
[0021] 2) After the reaction, the reaction solution was added with 2 mL of water, and then extracted with ethyl acetate for 3-5 times. The organic layer was concentrated and subjected to column chromatography to obtain a pure amide compound as a yellow liquid with a yield of 76%.
[0022] 1 H NMR(400MHz, CDCl 3 )δ7.45-7.36 (m, 5H), 3.77-3.46 (m, 8H). 13 C NMR(100MHz, CDCl 3 )δ170.5, 135.3, 129.9, 128.6, 127.1, 66.9 (2C), 48.1, 42.5.
example 2
[0024]
[0025] 1) Add 0.4mmol of m-methylphenylhydrazine hydrochloride and 3mol% of Pd(OAc) into a clean 25mL Schlenk tube 2 ,20mol% PPh 3 , 0.2mmol of Na 2 CO 3 , In CO:O 2 =3:1, inject 0.2 mmol of morpholine and a mixed solvent (dimethyl sulfoxide: dioxane = 0.1: 0.9 mL), react at 100° C. for 12 hours, and detect by TLC dot plate.
[0026] 2) After the reaction, the reaction solution was added with 2 mL of water, and then extracted with ethyl acetate for 3-5 times. The organic layer was concentrated and subjected to column chromatography to obtain a pure amide compound as a yellow liquid with a yield of 79%.
[0027] 1 H NMR(400MHz, CDCl 3 )δ7.31-7.16 (m, 4H), 3.73-3.45 (m, 8H), 2.37 (s, 3H).
[0028] 13 C NMR(100MHz, CDCl 3 )δ170.6, 138.5, 135.5, 130.6, 128.4, 127.7, 124.0, 66.9 (2C), 48.2, 42.4, 21.4.
example 3
[0030]
[0031] 1) Add 0.4mmol 4-tert-butylphenylhydrazine hydrochloride and 0.03mmol Pd(OAc) into a clean 25mL Schlenk tube 2 , 0.2mmol PPh 3 , 0.2mmol of Na 2 CO 3 , In CO:O 2 =3:1 in an atmosphere of 0.2 mmol of morpholine and a mixed solvent (dimethyl sulfoxide: dioxane = 0.1: 0.9 mL), react at 100° C. for 12 h, and TLC spot plate detection.
[0032] 2) After the completion of the reaction, the reaction solution was added with 2 mL of water, and then extracted with ethyl acetate for 3-5 times. The organic layer was concentrated and subjected to column chromatography to obtain a pure amide compound, a yellow solid, with a yield of 90%.
[0033] 1 H NMR(400MHz, CDCl 3 )δ7.42(d,J=8.3Hz,2H), 7.34(d,J=8.3Hz,2H), 3.69-3.51(m,8H),1.32(s,9H). 13 C NMR(100MHz, CDCl 3 )δ170.7, 153.2, 132.3, 127.0, 125.5, 66.9 (2C), 48.4, 42.4, 34.8, 31.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com