Method for DNA detection and quality control in fetus chromosome aneuploid detection
A technology of aneuploidy and quality control method, applied in the field of non-invasive prenatal screening and detection, which can solve the problems affecting the success rate of detection, incomplete separation of plasma, and unclear standards.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] A DNA detection and quality control method in the detection of fetal chromosomal aneuploidy, characterized in that it includes:
[0082] Step A: sample collection, preservation and quality control;
[0083] Step B: DNA extraction;
[0084] Step C: library construction;
[0085] Step D: library concentration determination and storage;
[0086] Step E: On-machine sequencing;
[0087] Step F: result analysis;
[0088] Wherein, the step A includes:
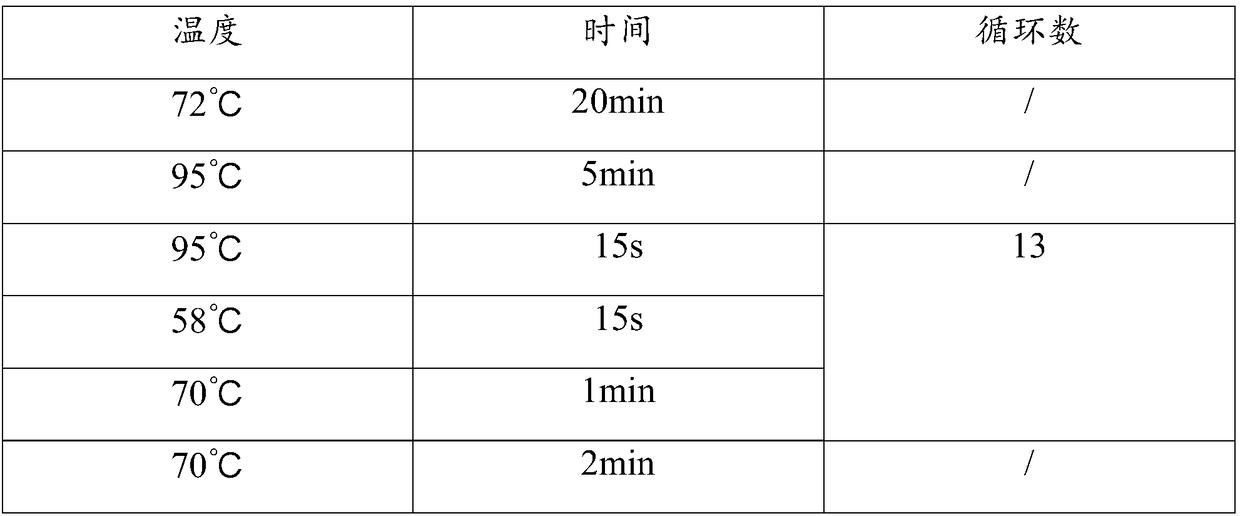
[0089] Sample collection: Blood collection tube 1 collects 5.0 mL of pregnant women’s peripheral blood. After the collection, gently invert the blood collection tube and place the blood collection tube in the refrigerator in time; blood collection tube 2 collects 10.0 mL of pregnant women’s peripheral blood. Preserve; pre-cool the low-speed centrifuge until the temperature is stable, put it into the blood collection tube for two minutes, absorb the supernatant plasma, transfer it to a 2.0mLEP tube placed on the ice box, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com