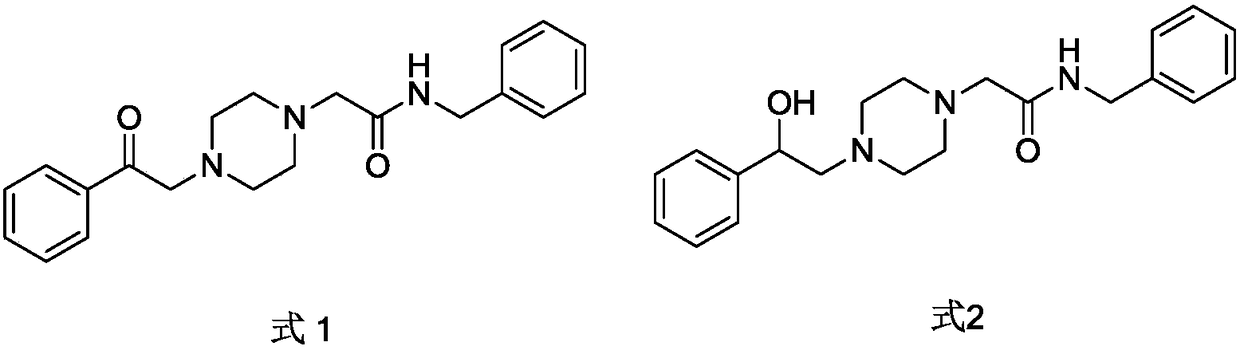
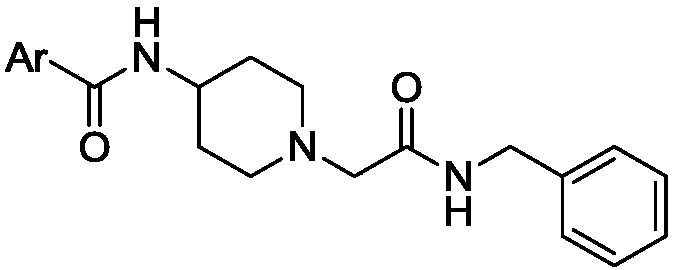
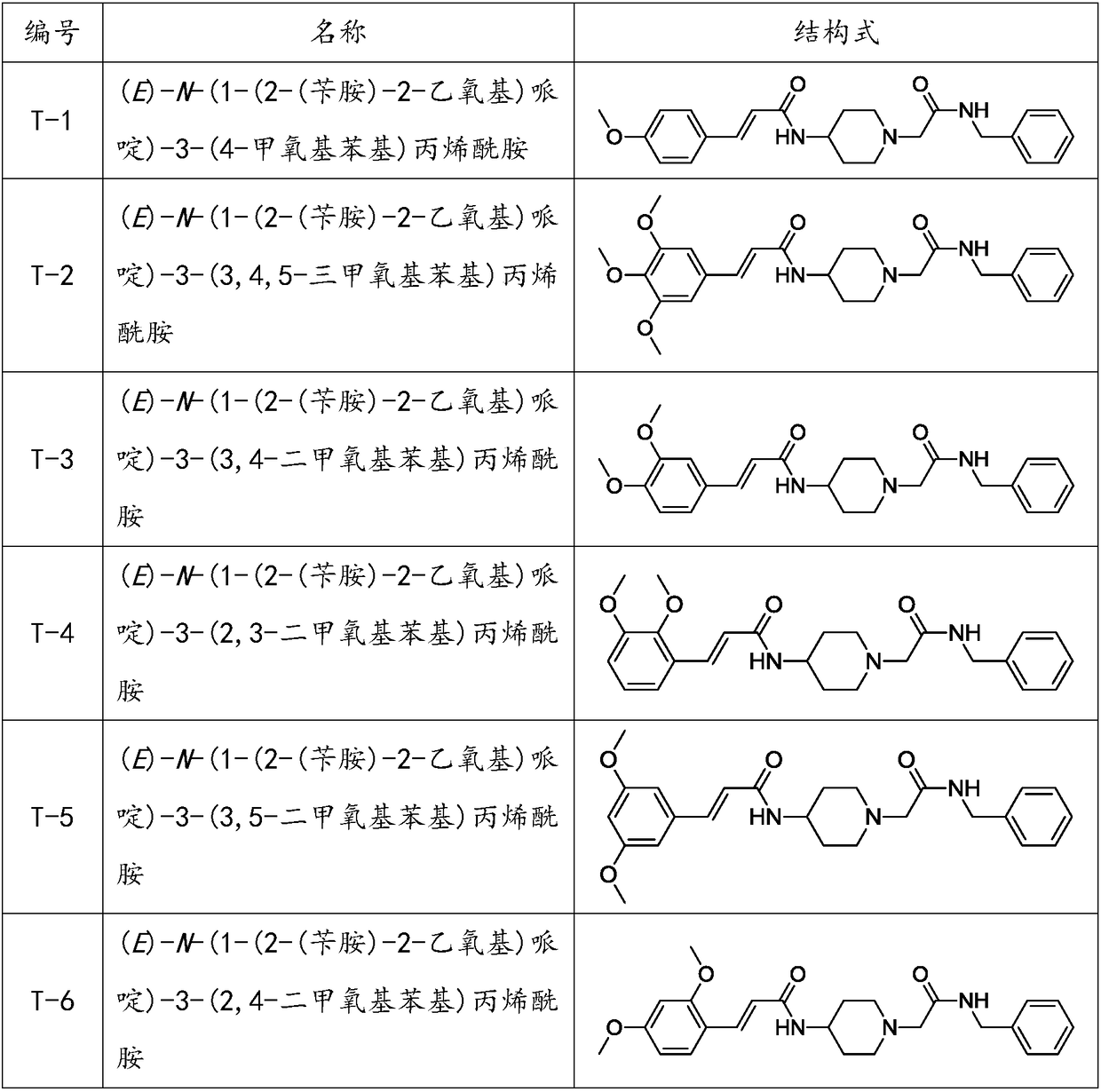
Benzylamine acetyl piperidine acylamide derivative as well as application of benzylamine acetyl piperidine acylamide derivative as cranial nerve protective agent
A technology of acetylbenzylamine piperidine amide and derivatives, applied in the application field of brain neuroprotective agents, which can solve the problems of high cardiotoxicity and weak activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: (E)-N-(1-(2-(benzylamine)-2-ethoxy)piperidine)-3-(4-methoxyphenyl)acrylamide (T- 1) Preparation of its salt
[0067] Using 4-methoxycinnamic acid as raw material, according to the general synthetic method, 1.45 g of the target product was obtained with a yield of 85%.
[0068] ESI-MS[M+H] + m / z=408.2; 1 H NMR (400MHz, DMSO-d6) δppm: 10.17(s, 1H), 9.30(t, J=8.0Hz, 1H), 8.38(d, J=8.0Hz, 1H), 7.50(d, J=12.0Hz ,2H),7.41(s,1H),7.37~7.25(m,5H),6.98(d,J=8.0Hz,2H),6.53(d,J=16.0Hz,1H),4.36(d,J= 4.0Hz, 2H), 4.15~4.00(m, 3H), 3.79(s, 3H), 3.51(d, J=12.0Hz, 2H), 3.25~3.18(m, 2H), 2.08~1.98(m, 2H ),1.88~1.79(m,2H).
[0069] Preparation of compound T-1 hydrochloride
[0070] Compound T-1 (0.3g) and 5% hydrochloric acid aqueous solution (0.8mmol) were added to ethanol (10mL), refluxed and dissolved, and a white solid was precipitated by cooling, which was filtered to obtain 0.3g of white T-1 hydrochloride solid.
[0071] Preparation of compound T-1 mesylate
[007...
Embodiment 2
[0077] Example 2: (E)-N-(1-(2-(benzylamine)-2-ethoxy)piperidine)-3-(3,4,5-trimethoxyphenyl)propene Preparation of amides (T-2) and their salts
[0078] Using 3,4,5-trimethoxycinnamic acid as a raw material, and operating according to the general synthetic method, 1.45 g of the target product was obtained with a yield of 85%.
[0079] ESI-MS[M+H] + m / z=468.1; 1 H NMR (400MHz, DMSO-d6) δppm: 10.08(s, 1H), 9.25(t, J=8.0Hz, 1H), 8.35(d, J=8.0Hz, 1H), 7.40(s, 1H), 7.37 ~7.26(m, 5H), 6.90(d, J=4.0Hz, 2H), 6.61(d, J=16.0Hz, 1H), 4.36(d, J=4.0Hz, 2H), 4.06~3.99(m, 3H), 3.81(s, 6H), 3.68(s, 3H), 3.52~3.47(m, 2H), 3.25~3.11(m, 2H), 1.99(s, 2H), 1.86~1.78(m, 2H) .
[0080] Preparation of compound T-2 hydrobromic acid salt
[0081] Using compound T-2 (2.0 mmol) and 5% hydrobromic acid aqueous solution (2.1 mmol) as raw materials, the preparation method of compound T-1 hydrobromide was used to obtain 0.9 g of white T-2 hydrobromide solid.
Embodiment 3
[0082] Example 3: (E)-N-(1-(2-(benzylamine)-2-ethoxy)piperidine)-3-(3,4-dimethoxyphenyl)acryloyl Preparation of amine (T-3) and its salt
[0083] Using 3,4-dimethoxycinnamic acid as raw material, according to the general method, the target product was obtained 1.45g, and the yield was 85%.
[0084] ESI-MS[M+H] + m / z=438.2; 1H NMR (400MHz, DMSO-d6) δppm: 10.06(s, 1H), 9.23(d, J=8.0Hz, 1H), 8.29(d, J=8.0Hz, 1H), 7.38( d, J=8.0Hz, 2H), 7.35~7.25(m, 5H), 7.13(t, J=8.0Hz, 2H), 6.99(d, J=8.0Hz, 1H), 6.53(d, J=12.0 Hz, 1H), 4.36(d, J=4.0Hz, 2H), 4.12~3.88(m, 3H), 3.78(d, J=4.0Hz, 6H), 3.50(d, J=12.0Hz, 2H), 3.20(q, J=12.0Hz, 2H), 2.06~1.98(m, 2H), 1.85~1.77(m, 2H).
[0085] Preparation of Compound T-3 Fumarate
[0086] Using compound T-3 (2.3 mmol) and fumaric acid (2.4 mmol) as raw materials, the preparation method of compound T-1 hydrobromide was adopted to obtain 1.0 g of white solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com