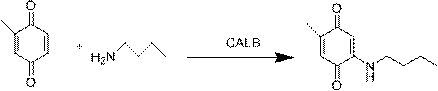
Method for preparing methylbutylamine p-benzoquinone through lipase catalysis
A technology for the catalytic preparation of methylbutylamine, applied in biochemical equipment and methods, enzymes, hydrolytic enzymes, etc., can solve the problems of high separation cost, difficult separation and purification, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 10ml brown reaction bottle, stir with magnet, add 2ml of citric acid buffer solution with pH 7.0, which contains 3-5% methanol, methyl-p-benzoquinone and butylamine are fed in equimolar ratios, the concentration of butylamine is 0.05-0.1mM, Add immobilized Candida antarctica lipase B, Novozme 435, the amount of enzyme added is 5-10U / mL, the reaction temperature is 38-42°C, and N 2 After 3 minutes, seal the bottle cap and start the reaction, the reaction time is 20-24h. After the reaction was finished, samples were taken for liquid chromatography analysis, and the liquid phase detection results: the reaction conversion rate was 99-100%, the product 2-methyl-5-butylamine p-benzoquinone yield was 94-95%, and other substituted products produced The rate is less than 2.5%.
[0015] Liquid phase detection conditions: Agilent 1200 liquid chromatograph, C18 reverse phase column, 250mm, mobile phase: methanol / water=80 / 20, product 2-methyl-5-butylamine-p-benzoquinone retention ...
Embodiment 2
[0017] 100ml brown reaction bottle, stir with magnet, add 20ml of citric acid buffer solution with pH 7.0, which contains 3-5% methanol, methyl p-benzoquinone and butylamine are fed in equimolar ratios, the concentration of butylamine is 0.05-0.1mM, Add immobilized Candida antarctica lipase B, Novozme 435, the amount of enzyme added is 5-10U / mL, the reaction temperature is 38-42°C, and N 2 After 5 minutes, close the bottle cap and start the reaction, the reaction time is 20-24h. After the reaction, take a sample for liquid chromatography analysis, filter out the immobilized enzyme, and the immobilized enzyme can be reused. The filtrate was extracted with acetone, the organic phase was separated, and the acetone was evaporated to obtain the product 2-methyl-5-butylamino-p-benzoquinone. The liquid phase detection results: the reaction conversion rate was 99-100%, and the product 2-methyl-5- The yield of butylamine-p-benzoquinone is 94-95%, and the yield of other substituted pr...
Embodiment 3
[0020] 1000ml brown reaction bottle, stir with magnet, add 500ml of citric acid buffer solution with pH 7.0, which contains 3% methanol, methyl-p-benzoquinone and butylamine are fed equimolarly, the concentration of butylamine is 0.05mM, add immobilized Antarctic Candida lipase B, Novozme 435, enzyme dosage 5U / mL, reaction temperature 42°C, N 2 After 15 minutes, seal the bottle cap and start the reaction, the reaction time is 20h. After the reaction, take a sample for liquid chromatography analysis, filter out the immobilized enzyme, and the immobilized enzyme can be reused. The filtrate was extracted with acetone, the organic phase was separated, and the acetone was evaporated to obtain the product 2-methyl-5-butylamine-p-benzoquinone. The liquid phase detection result: the reaction conversion rate was 99%, and the product 2-methyl-5-butylamine The yield of p-benzoquinone was 94%, and the yield of other substituted products was less than 2.5%.
[0021] The detection condit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com