Application of carnosol in preparation of medicines for preventing and treating experimental autoimmune encephalomyelitis
An autoimmune, carnosol-based technology for biomedical and pharmaceutical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Experimental treatment
[0046] (1) EAE model preparation
[0047] Eight-week-old female C57BL / 6J mice were purchased from the Air Force Military Medical University of the Chinese People's Liberation Army (Xi'an, China). myelin oligodendrocyte glycoprotein peptide 35-55 (MOG 35-55 ), purchased from Genescript Company, pertussis toxin, purchased from Sigma-Aldrich Company, complete Freund's adjuvant containing Mycobacterium tuberculosis, purchased from BD Difco Company.
[0048] Dissolve MOG with PBS 35-55 The polypeptide is then mixed with an equal volume of complete Freund's adjuvant (containing 5 mg / ml Mycobacterium tuberculosis), and pushed with a glass syringe until a water-in-oil white antigen emulsion is formed.
[0049] Mice were immunized at two sites on the back, and diluted pertussis solution (200 ng / mouse) was injected intraperitoneally on the day of immunization and 2 days later, respectively.
[0050] Grouping and dosing:
[0051] ①PBS-treated cont...
Embodiment 2
[0071] 1. Experimental Treatment
[0072] The same modeling administration as in Example 1, MNC was prepared from the spleen and lymph nodes of IL-17A-IRES-GFP mice (C57BL / 6J) on the 10th day after modeling to study the pathogenicity of carnosol on Th17 cells Sexuality, cells were cultured with PBS and carnosol under Th17 differentiation conditions, and treated with MOG 35-55 (25 μg / ml), IL-2 (2ng / ml) and IL-23 (10ng / ml) stimulation. After three days in culture, CD4+ T cells were isolated. The cells (4×10 6 cells) injected into C57BL / 6J recipient mice. After 20 days, the mice were sacrificed, and different groups of brain tissues were collected for immunohistochemical staining.
[0073] 2. Experimental results
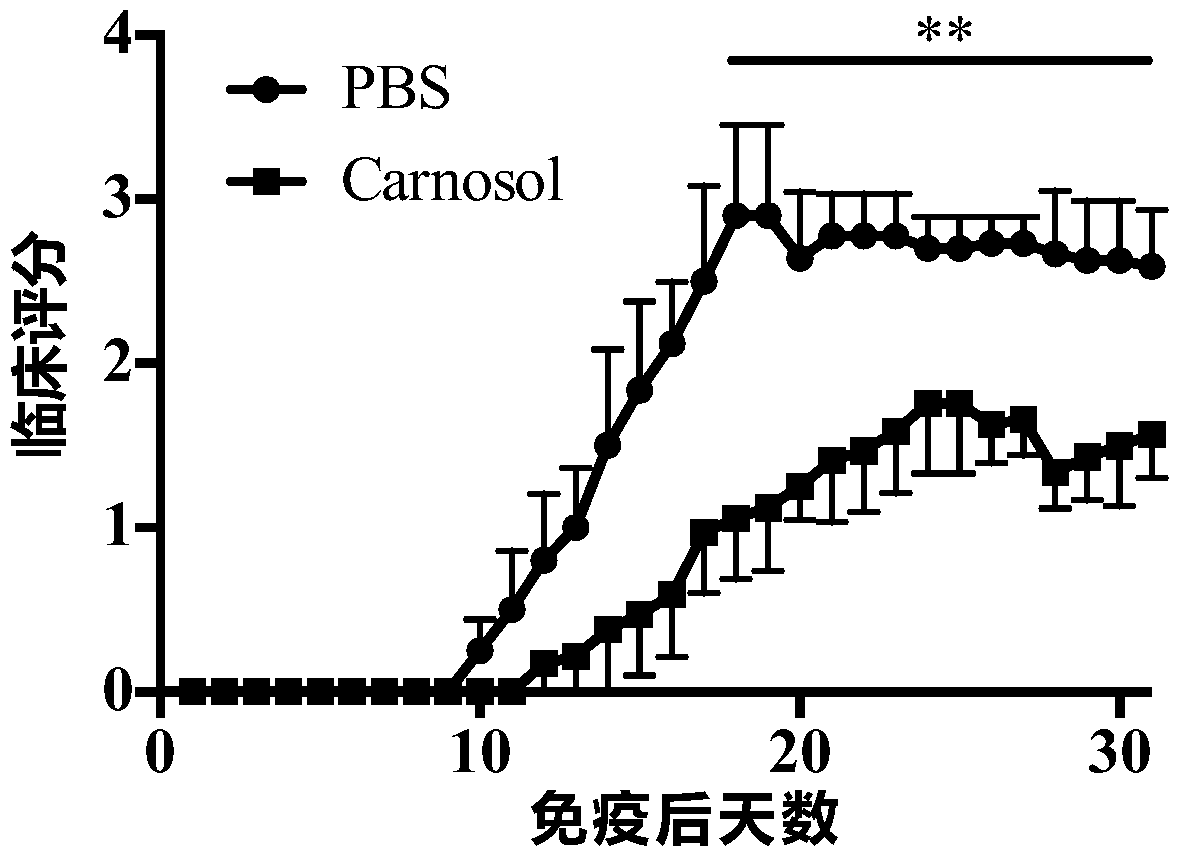
[0074] (1) Compared with the PBS treatment group, the Th17 cells treated with carnosol significantly reduced the severity of clinical disease after transfer (p Figure 10 ).
[0075] (2) Immunofluorescence staining results of the mice sacrificed after the 20th ...
Embodiment 3
[0077] 1. Experimental Treatment
[0078] (1) C57BL / 6J mice aged 8-10 weeks were treated with MOG 35-55 After immunization, the mice were treated from day 25 and injected with PBS and carnosol for clinical scoring.
[0079] (2) On the 60th day of treatment, the spinal cord and lumbosacral enlargement of PBS and EAE mice were excised for immunohistochemistry and MBP staining, and quantitatively analyzed with Imag-Pro.
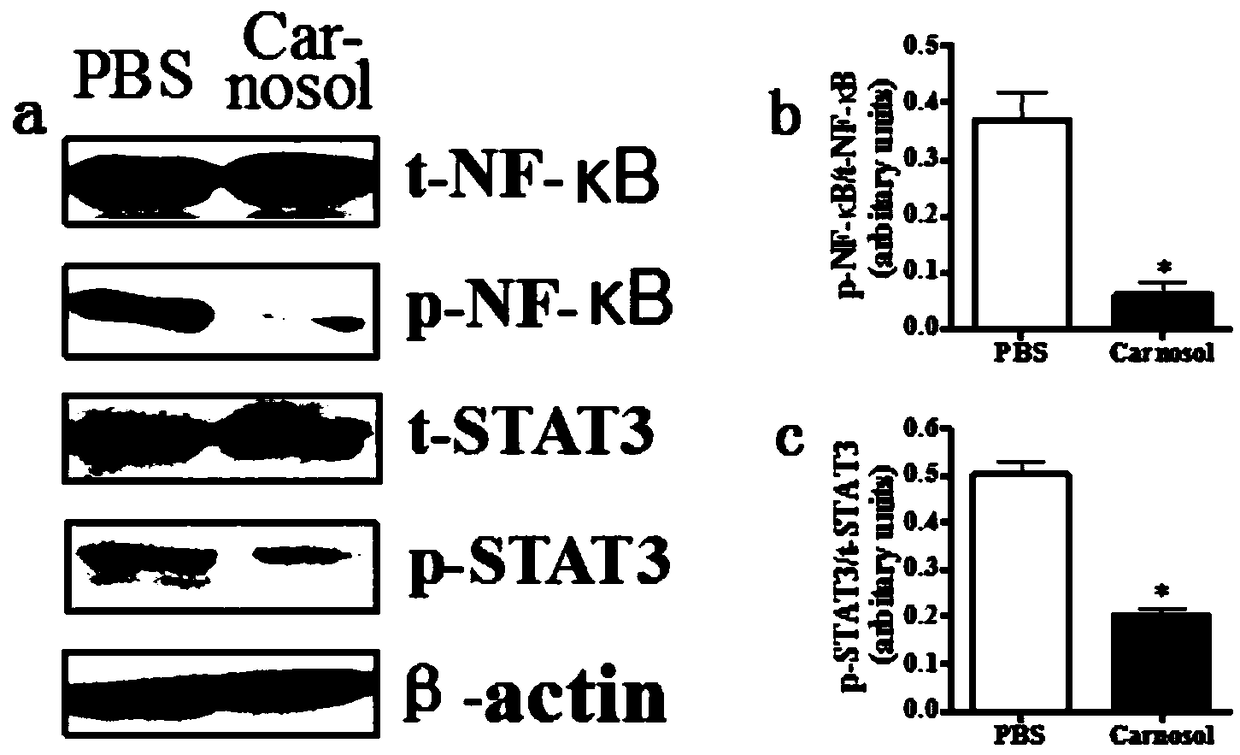
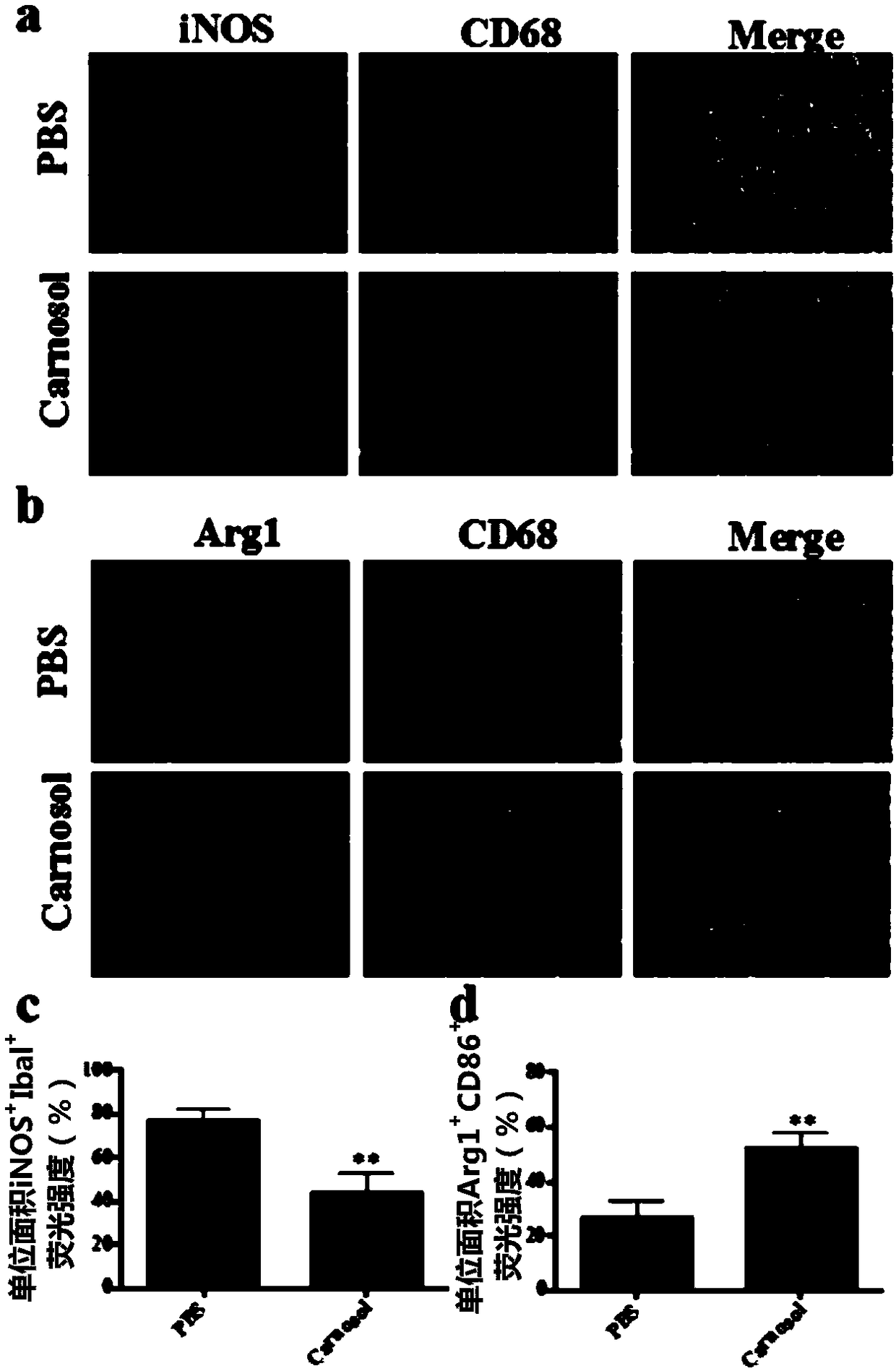
[0080] (3) Staining of pro-inflammatory (M1) and immunomodulatory (M2) markers of infiltrating macrophages and innate microglia in the mouse spinal cord to assess the effect of carnosol on these cells in the spinal cord.
[0081] (4) Prepare primary microglial cells from newborn C57BL / 6 mice, stimulate with lipopolysaccharide (LPS, 100ng / ml) and treat with different concentrations of carnosol for 2 days, and detect TNF in the supernatant by ELISA -α expression, and the cells were collected to detect the expression of related genes by real-time fluorescent quan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com