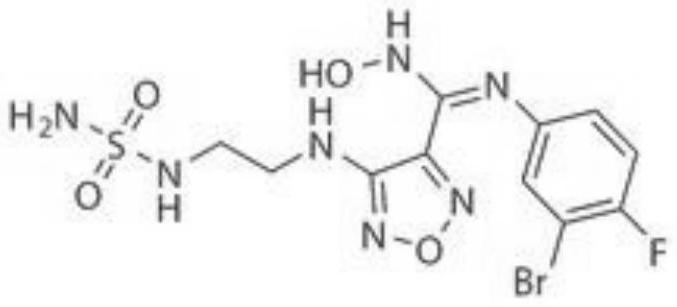
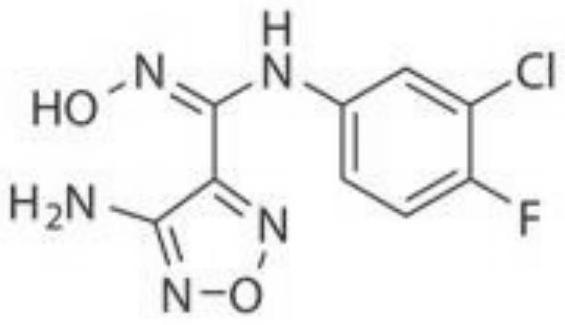
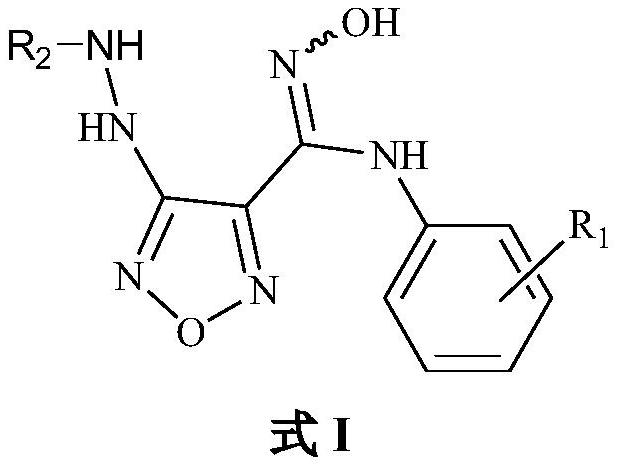
A hydrazine-containing indoleamine 2,3-bisoxidase inhibitor
A technology of group and alkyl group, applied in the field of IDO inhibitor containing hydrazine group and its preparation, can solve the problem of inability to directly regulate the immune response system of immune cells and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1 Synthesis of compound I-1
[0080]
[0081] The first step. 5.0g of compound 2 was dissolved in 25.0ml of ethyl acetate (EA), 4.7g of p-chloroaniline was added in batches, TLC detected the reaction until the reaction was complete, 0.5ml of triethylamine was added dropwise to quench the reaction, and ethyl acetate was added. Ester and water, separated, the organic phase was washed with saturated NaCl solution and anhydrous NaSO 4 Dry, filter with suction, concentrate the filtrate under reduced pressure, and purify by column chromatography to obtain 6.2 g of off-white solid compound 15;
[0082] The second step. 5.0g of compound 15 was dissolved in 100ml of tetrahydrofuran (THF), 3.2g of N,N-carbonyldiimidazole was added, the temperature was raised to 70°C and the reaction was performed until TLC detected that the reaction of the raw materials was complete, the reaction was stopped, the temperature was cooled to room temperature, and the pressure was reduced...
Embodiment 2
[0087] Example 2 Synthesis of compound I-38
[0088]
[0089] The first step. Take 5.0g malononitrile, add 10ml water-soluble clear, then cool down to below 0°C, add 6.0g sodium nitrite, stir for 1.0h, turn off the refrigeration, add 1.35ml 4N hydrochloric acid, move to room temperature and stir for 2.0h , then cooled to below 0°C, slowly added dropwise 15.5g of 50% hydroxylamine aqueous solution, heated to reflux after the dropwise addition, refluxed for 3 hours, cooled to 6°C, stirred overnight, and below 0°C, slowly added dropwise dilute hydrochloric acid to adjust pH =5 or so, a large amount of solid was precipitated, and 10.0 g of compound 1 was obtained by suction filtration;
[0090] Step 2. Dissolve 10.0g of compound 1 in 50ml of acetic acid, then add 100ml of water, 38ml of 6N HCl and 13.0g of sodium chloride, after dissolving, drop to below 0°C, slowly add 5.3g of sodium nitrite / 15ml dropwise Aqueous solution, and control the temperature below 0 °C, continue to...
Embodiment 3
[0096] Example 3 Synthesis of compound I-34:
[0097]
[0098] The first step. Dissolve 280mg of compound 11 in 5ml of tetrahydrofuran, add 120mg of triethylamine and then cool down to below 0°C, slowly add 290mg of p-trifluoromethoxybenzenesulfonyl chloride, TLC detects that the reaction of the raw materials is complete, add 25ml of water to quench , extracted with ethyl acetate, retained the organic phase, washed the organic phase with saturated NaCl solution and dried with anhydrous NaSO , suction filtered, and the filtrate was concentrated under reduced pressure to obtain the crude compound 12, which was directly put into the next step;
[0099] The second step. The above-mentioned compound 12 is dissolved in 1ml of tetrahydrofuran, and 1ml of 85% hydrazine hydrate is added. TLC detects that the reaction of the raw materials is complete, and most of the THF is removed under reduced pressure. Chromatography yielded 50 mg of compound 1-34 as an off-white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com