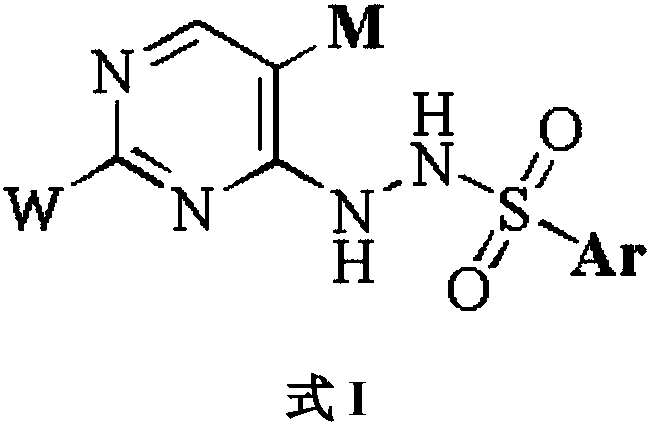
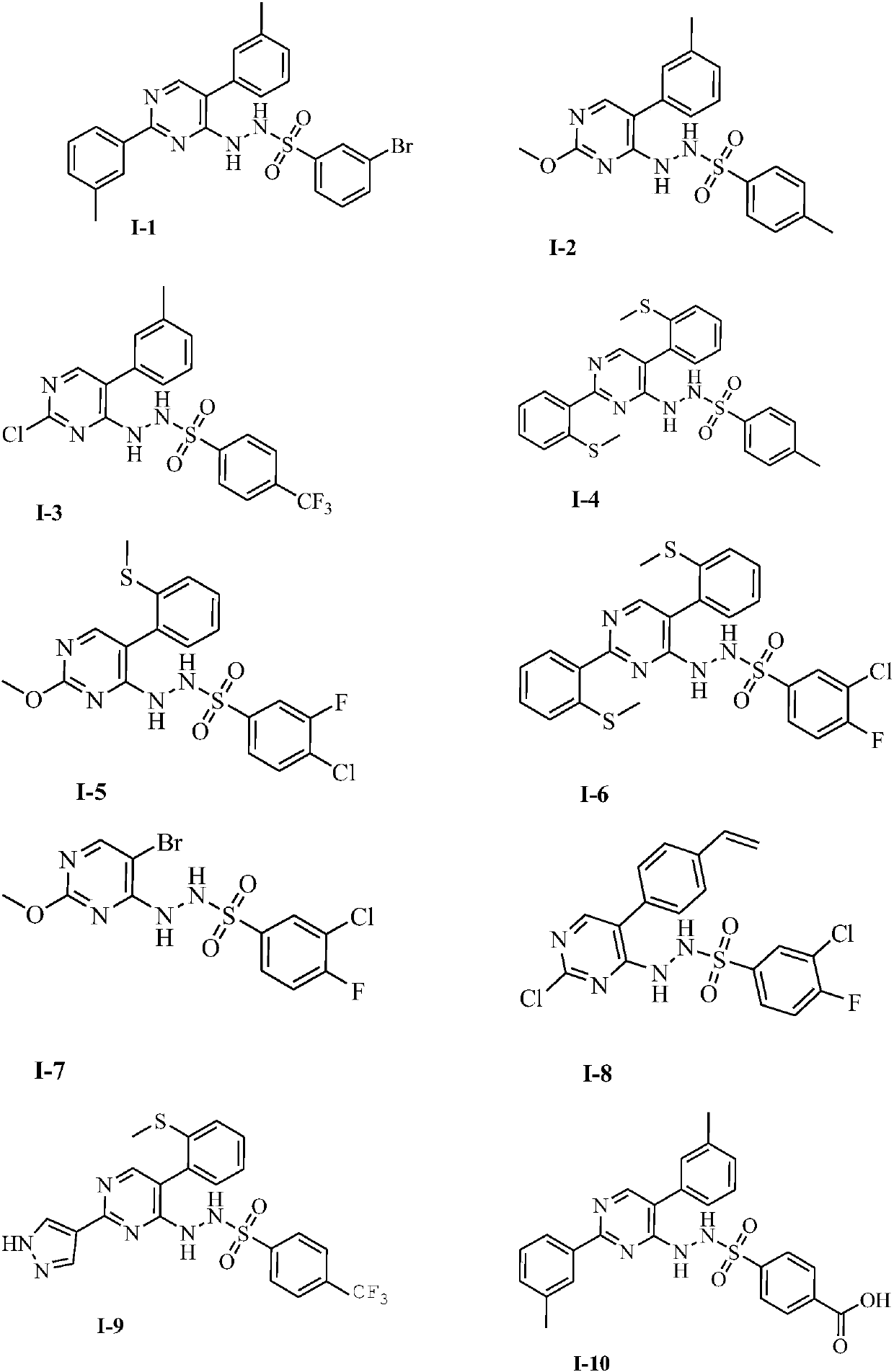
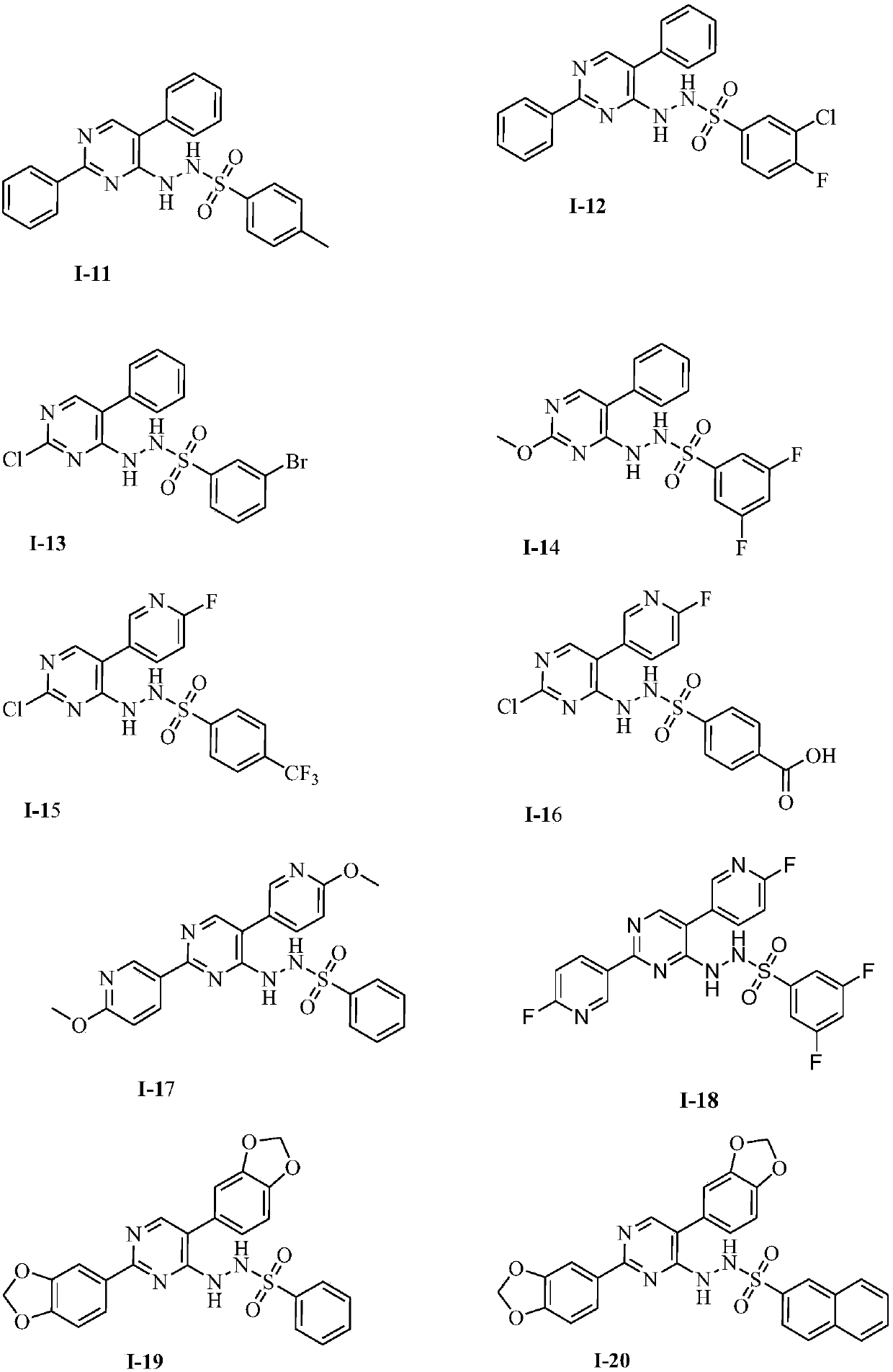
Novel IDO (indoleamine 2,3-dioxygenase) inhibitor
A compound and pharmaceutical technology, applied in the field of IDO inhibitors and their preparation, can solve the problems of unsatisfactory inhibitory efficacy of IDO inhibitors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The preparation of embodiment 1 compound I-1
[0062]
[0063] Step 1: Synthesis of Compound 1
[0064] Add 5.05g of 5-bromo-2,4-dichloropyrimidine to a 100ml one-mouth bottle successively, add 25ml of ethanol to dissolve it, place it at 0°C, add 2.25g of triethylamine, weigh 3.50g of Boc hydrazine, and use 50ml After the ethanol is dissolved, drop it into the above reaction solution at low temperature. After the dropwise addition, transfer it to room temperature and stir for 2 hours. TLC detects that the raw material has reacted completely. Spin off the ethanol, pour it into water, add EA for extraction, and wash once with citric acid aqueous solution. , washed twice with saturated sodium chloride, extracted once with water, combined the organic phases, dried and spin-dried to obtain compound 1 as a pale yellow solid.
[0065] The second step: the synthesis of compound 2
[0066] Add 0.5g compound 1, 0.74g phenylboronic acid, 0.28g Pd(dppf)Cl to the 100ml one-mout...
Embodiment 2
[0074] The preparation of embodiment 2 compound 1-2
[0075]
[0076] The first step: the synthesis of compound 6
[0077] Add 0.20g of compound 5 to a 100ml one-mouth bottle in turn, add 10ml of methanol to dissolve it, then add 5ml of concentrated hydrochloric acid, react at 50°C for half an hour, TLC detects that the raw materials have reacted completely, spin off the methanol, pour into water and add ammonia water to adjust to a weak base nature, added EA to extract, the organic phase was washed twice with saturated sodium chloride, the organic phase was dried and spin-dried to obtain a brownish black oily liquid, and compound 6 was obtained by column chromatography.
[0078] The second step: the synthesis of compound I-2
[0079] Add 0.21g of compound 6 to a 100ml single-necked bottle in turn, add 10ml of DMF to dissolve it, then add 0.20g of p-toluenesulfonyl chloride dissolved in 2ml of DMF dropwise at low temperature, after the addition is completed, stir at low te...
Embodiment 3
[0082] The preparation of embodiment 3 compound 1-3
[0083]
[0084] The first step: the synthesis of compound 5
[0085] Add 1.01g compound 1, 1.05g m-methylphenylboronic acid, 0.18g Pd(dppf)Cl to 100ml one-port bottle 2 , 1.28g of potassium carbonate, 30ml of 1,4-dioxane and 5ml of water, reacted at 100°C for 12 hours under the protection of nitrogen, TLC detected that the raw materials were completely reacted, poured into water, extracted with EA, and the organic phase was saturated with chlorinated Washed twice with sodium, extracted once with water, combined the organic phases, dried and spin-dried, and separated by column chromatography to obtain 0.14 g of compound 5 as an off-white solid.
[0086] The second step: the synthesis of compound 8
[0087] Add 0.20g of compound 5 to a 100ml one-mouth bottle in turn, add 10ml THF to dissolve it, then add 3ml concentrated hydrochloric acid, react at room temperature for 2 hours, TLC detects that the raw materials have rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com