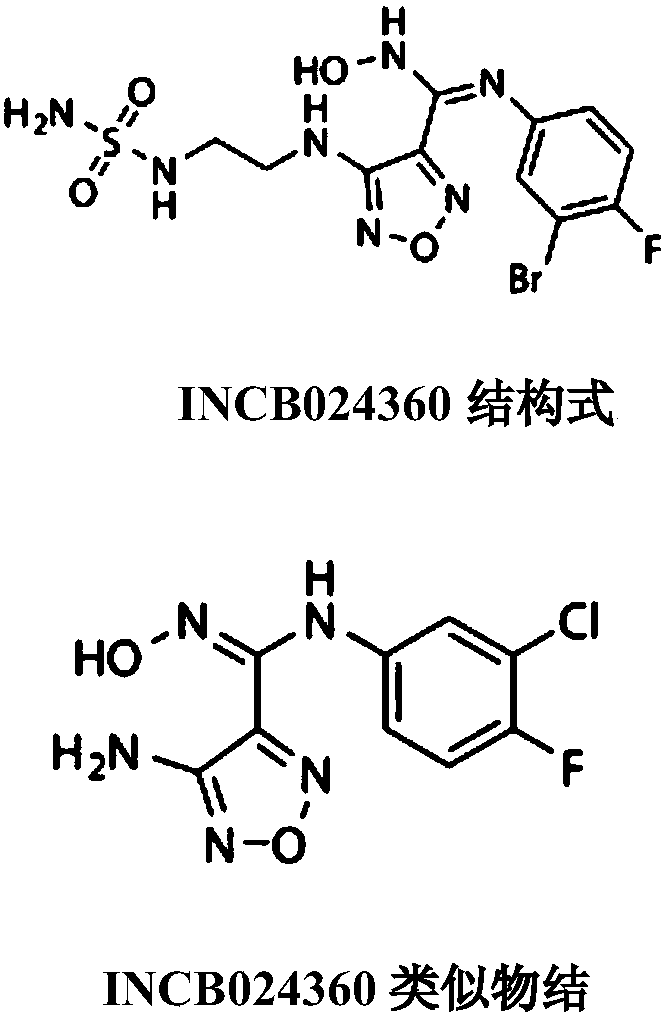
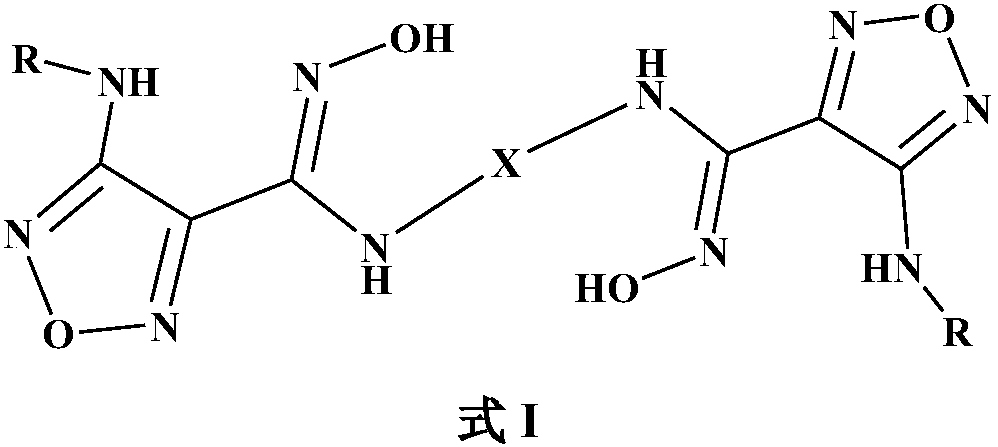
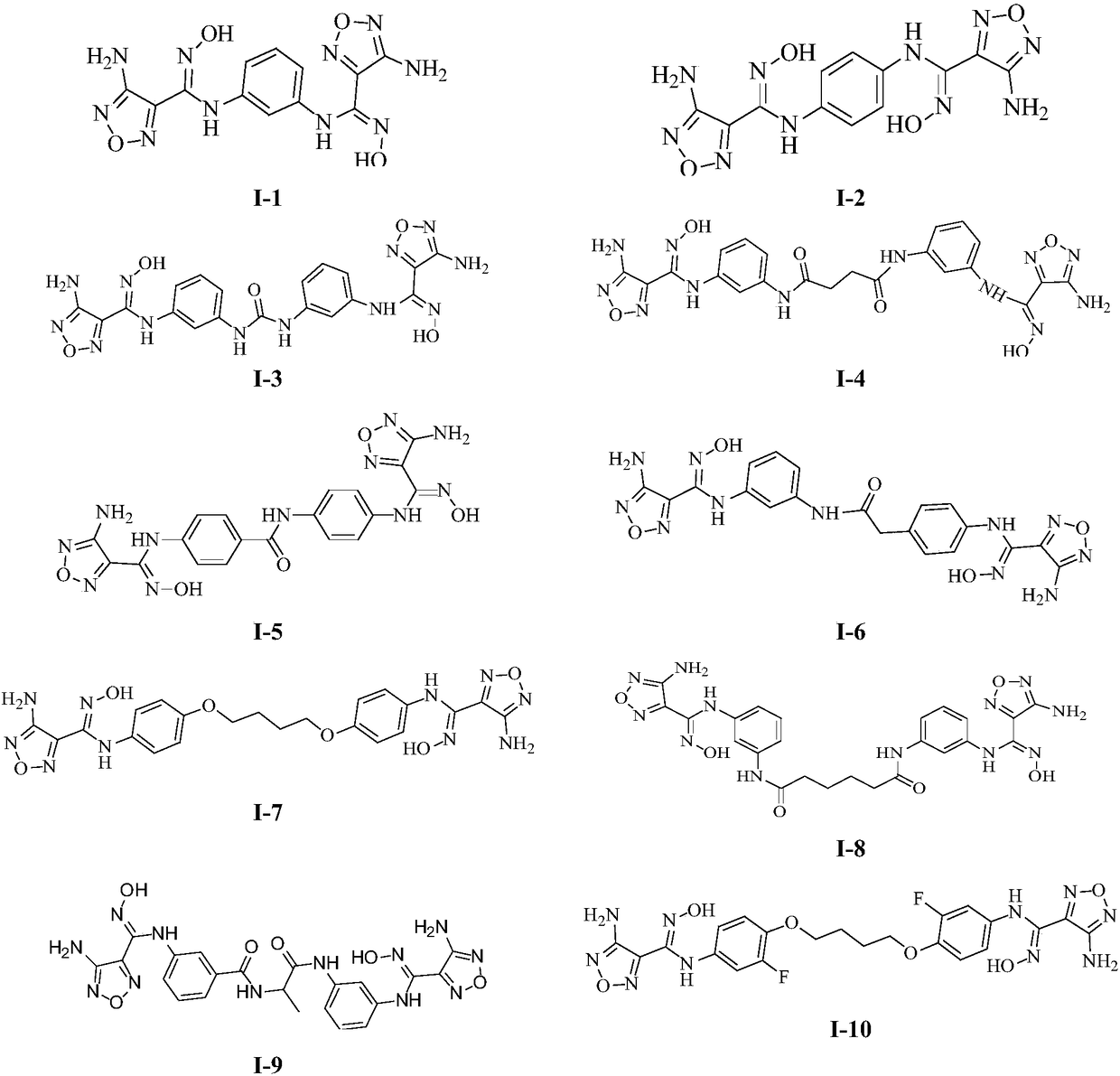
Novel indoleamine 2,3-dioxygenase inhibitor
A compound and aryl technology, applied in the field of efficient IDO inhibitor and its preparation, can solve the problem of inability to directly regulate the immune cell immune response system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Example 1 Synthesis of Compound I-1
[0143]
[0144] Take 0.3g of m-phenylenediamine, add 10ml of ethyl acetate to dissolve it, add 0.9g of compound 1, stir at room temperature, TLC detects that the raw materials have reacted completely, add a few drops of triethylamine to the reaction solution, add 50ml of water, and dissolve with ethyl acetate. Ester extraction (20ml*3), the organic phases were combined, dried over anhydrous sodium sulfate and then reduced to dryness under reduced pressure, and 40 mg of yellow solid I-1 was obtained by column chromatography.
Embodiment 2
[0145] Embodiment 2 compound I-2 is synthesized
[0146]
[0147] Take 0.3g of p-phenylenediamine, add 10ml of ethyl acetate to dissolve it, add 0.9g of compound 1, stir at room temperature, TLC detects that the raw material has reacted completely, add a few drops of triethylamine to the reaction solution, add 50ml of water, and dissolve with ethyl acetate. Ester extraction (20ml*3), the organic phases were combined, dried over anhydrous sodium sulfate and then reduced to dryness under reduced pressure, and 30mg of yellow solid I-2 was obtained by column chromatography.
Embodiment 3
[0148] Embodiment 3 compound I-16 is synthesized
[0149]
[0150]Step 1. Get 405mg p-aminophenylboronic acid, 400mg 2-amino-5-bromo-3-methoxypyrazine, 552mg potassium carbonate, 30mg tetrakistriphenylphosphine palladium, 10ml DMF and 1ml water, replace nitrogen, and heat up to React at 100°C overnight, TLC detects that the reaction of the raw materials is complete, cool down to room temperature, add water and ethyl acetate, separate the layers, dry the organic phase over anhydrous sodium sulfate and reduce to dryness under reduced pressure, and obtain 100 mg of compound I-16-1 by column chromatography .
[0151] Step 2. Take 100mg of compound I-16-1, add 10ml of DMF to dissolve it, add 190mg of compound 1, stir at room temperature, TLC detects that the raw materials have reacted completely, add a few drops of triethylamine dropwise to the reaction solution, add 50ml of water, and dissolve with acetic acid Extract with ethyl ester (20ml*3), combine the organic phases, dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com