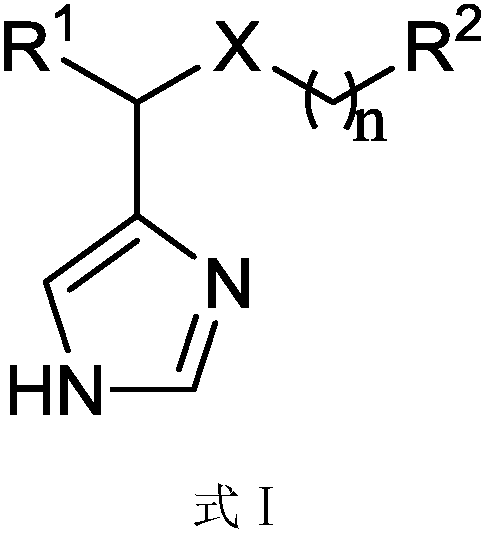
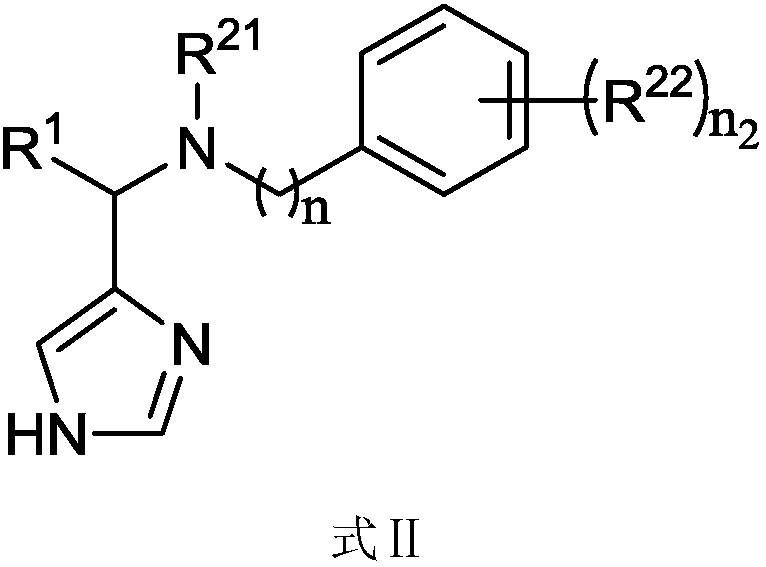
Imidazole methylamine derivatives having activity of indoleamine-2, 3-dioxygenase (IDO) inhibitor, and synthesis method of imidazole methylamine derivatives
A solvate, compound technology, applied in the field of compound medicine, can solve problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
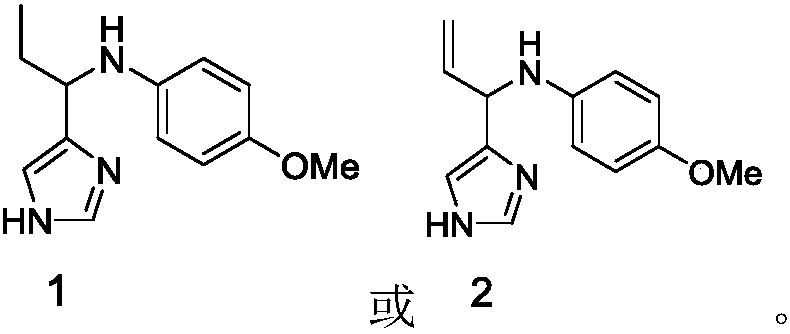
[0105] Embodiment 1, the synthesis of N-(1-(1H-imidazol-4-yl) propyl)-4-methoxyaniline (compound 1)
[0106]
[0107] (1) Synthesis of Intermediate 1b
[0108] First, 4-imidazole formaldehyde (1a, 960mg, 10mmol) was added to the reaction flask, placed under an ice-water bath, and after 10min, triethylamine (TEA) (278μl, 2.0mmol) was added to the reaction flask, and the mixture Stir for 5 min under ice-water bath. Then di-tert-butyl dicarbonate (2.4g, 1.0mmol) was added to the reaction flask, and the reaction was moved to room temperature for overnight reaction. After TLC detected that the reaction was complete, the solvent was spin-dried, and alumina column chromatography (PE:EA =2:1) to obtain intermediate 1b.
[0109] (2) Synthesis of Intermediate 1c
[0110]Then intermediate 1b (980.4mg, 5.0mmol) was placed in a three-necked flask, ventilated with argon, added cooled redistilled tetrahydrofuran (20ml) and cooled at -78°C for 2h, then poured into the reaction flask w...
Embodiment 2
[0120] Embodiment 2, the synthesis of N-(1-(1H-imidazol-4-yl) allyl)-4-methoxyaniline (compound 2)
[0121]
[0122] First, 2b was synthesized by using the raw material intermediate 1b and vinylmagnesium bromide according to the synthesis method of 1c.
[0123] Using 2b and acetyl chloride, 2c was synthesized according to the synthesis method of 1d.
[0124] Finally, the target compound 2 (white solid) was synthesized according to the synthesis method of compound 1 using 2c and raw material p-aminoanisole, with a total yield of 21%.
[0125] Compound 2 1 H NMR and 13 The C NMR data are as follows:
[0126] 1 HNMR(400MHz,DMSO)δ11.92(brs,1H),7.58(s,1H),6.89(s,1H),6.69(d,J=9.00Hz,2H),6.62(d,J=9.04Hz, 2H),6.03-5.95(m,1H),5.36(brs,1H),5.22(d,J=17.12Hz,2H),5.09(d,J=22.16Hz,2H),4.89(s,1H), 3.63(s,3H).
[0127] 13 C NMR (100MHz, DMSO): 151.3, 142.6, 139.9, 135.4, 115.0, 114.8, 114.7. ESIHRMS exact mass calcd.for (C 13 h 15 N 3 O-H) - requires m / z 228.1142, found m / z 228...
Embodiment 3
[0128] Example 3, 1-(1H-imidazol-4-yl)-N-methyl-1-phenylmethanamine
[0129]
[0130] First, use intermediate 1b and phenylmagnesium bromide to synthesize 3b according to the synthesis method of 1c, then use 3b and acetyl chloride to synthesize 3c according to the synthesis method of 1d, and finally use 3c and raw material methylamine aqueous solution to synthesize the target according to the synthesis method of compound 1 Compound 3 (transparent viscous liquid), the total yield is 12%.
[0131] Compound 3 1 H NMR and 13 The C NMR data are as follows:
[0132] 1 H NMR (400MHz, CDCl 3 )δ7.49(s,1H),7.29(q,J=7.9Hz,1H),7.19(d,J=8.0Hz,1H),7.13(d,J=10.0Hz,1H),6.95(td, J=8.4,2.4Hz,1H),6.71(s,1H),5.20(s,1H),4.30(s,2H)ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com