Method for recycling epoxy chloropropane based on epoxy chloropropane light component
A technology of epichlorohydrin and light components, which is applied in the field of chemical industry, can solve the problems of difficult handling of epichlorohydrin light components, achieve the goals of reducing environmental pollution and harm to human body, improving utilization rate and reducing production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
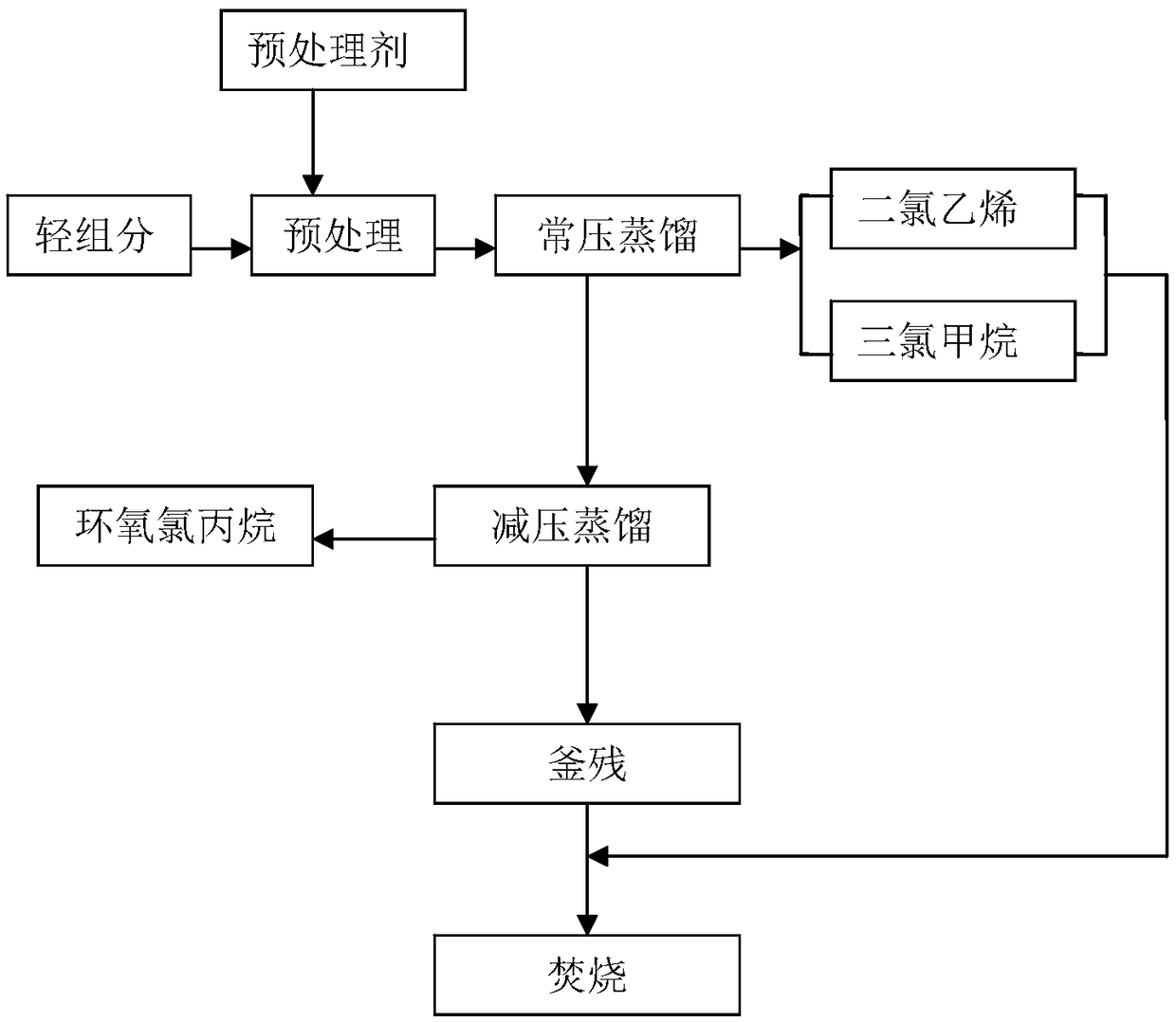
[0021] Take 100 g of the epichlorohydrin light component and put it into a 250 mL three-necked flask, add 4 g of triethanolamine, and react at room temperature for 15 min under magnetic stirring; then distill at a top temperature of 62°C for 20 min under normal pressure, and collect dichloroethylene and chloroform. The liquid is discharged, and its mass is weighed to be 39.1g; when no distillate is evaporated, it is changed to vacuum distillation, and the vacuum degree is -0.085MPa, and the temperature is 62°C. Distillate under reduced pressure, collect epichlorohydrin distillate, When no epichlorohydrin distillate evaporates, stop the distillation, and then distill under reduced pressure for 15 minutes to obtain 45.5 g of epichlorohydrin; the raffinate is cooled to 30 °C and distilled together with dichloroethylene and chloroform distillate Sent to the incinerator for incineration. The yield of epichlorohydrin is 95.20%, and the purity is 97.5%.
Embodiment 2
[0023] Take 100 g of the epichlorohydrin light component and put it into a 250 mL three-necked flask, add 4 g of triethylamine, and react at room temperature for 12 min under magnetic stirring; then distill under atmospheric pressure at a top temperature of 64 ° C for 25 min to collect dichloroethylene and chloroform Distillate, weighing its mass is 38.2g; when no distillate evaporates, change to vacuum distillation, vacuum distillation at -0.085MPa, temperature 64°C, collect epichlorohydrin distillate , when no epichlorohydrin distillate evaporates, stop the distillation, and then distill under reduced pressure for 12min to obtain 46.0g epichlorohydrin; Sent together to the incinerator for incineration. The yield of epichlorohydrin is 95.55%, and the purity is 96.8%.
Embodiment 3
[0025] Take 100 g of the epichlorohydrin light component and put it into a 250 mL three-necked flask, add 2 g of triethanolamine, and react at room temperature for 15 min under magnetic stirring; then distill under atmospheric pressure at a top temperature of 65 ° C for 15 min, and collect dichloroethylene and chloroform. The liquid is discharged, and its mass is weighed to be 37.9g; when no distillate is evaporated, it is changed to vacuum distillation, and the vacuum degree is -0.083MPa, and the temperature is 65°C. Distillate under reduced pressure, collect epichlorohydrin distillate, When no epichlorohydrin distillate evaporates, stop the distillation, and then distill under reduced pressure for 20 minutes to obtain 44.2 g of epichlorohydrin; the raffinate is cooled to 30 °C and distilled together with dichloroethylene and chloroform distillate Sent to the incinerator for incineration. The yield of epichlorohydrin is 92.29%, and the purity is 97.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com