A comprehensive identification method for the primary structure of intact n-glycoprotein
An identification method and technology for glycoproteins, which can be used in instruments, analytical materials, biomaterial analysis, etc., can solve the problems of limited structural information of glycoproteins, difficult to break, stability effects, etc., achieve simple sample processing steps, and comprehensively characterize glycoproteins. , the effect of comprehensive data information
Active Publication Date: 2020-06-02
TONGJI UNIV
View PDF6 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The above two methods are cumbersome and time-consuming, and are very prone to missed cuts; the obtained glycoprotein structure information is also limited
Since the polysaccharide structure modified by protein glycosylation has many connections and branched isomers, there is great structural heterogeneity, which brings great difficulties to characterize its structure in depth; other studies have shown that glycosylation affects the stability of proteins Also has an effect, the amino acid sequence around the glycosylated modification site is more difficult to break than the same sequence without glycosylation (for example, RNase B and RNase A) (Bourgoin-Voillard, S.; Leymarie, N.; Costello, C.E., Top-downtandem mass spectrometry on RNase A and B using a Qh / FT-ICR hybrid massspectrometer. Proteomics 2014, 14(10), 1174-1184.), so it is also important for the characterization of the protein backbone and the positioning of glycosylation sites raised a challenge
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
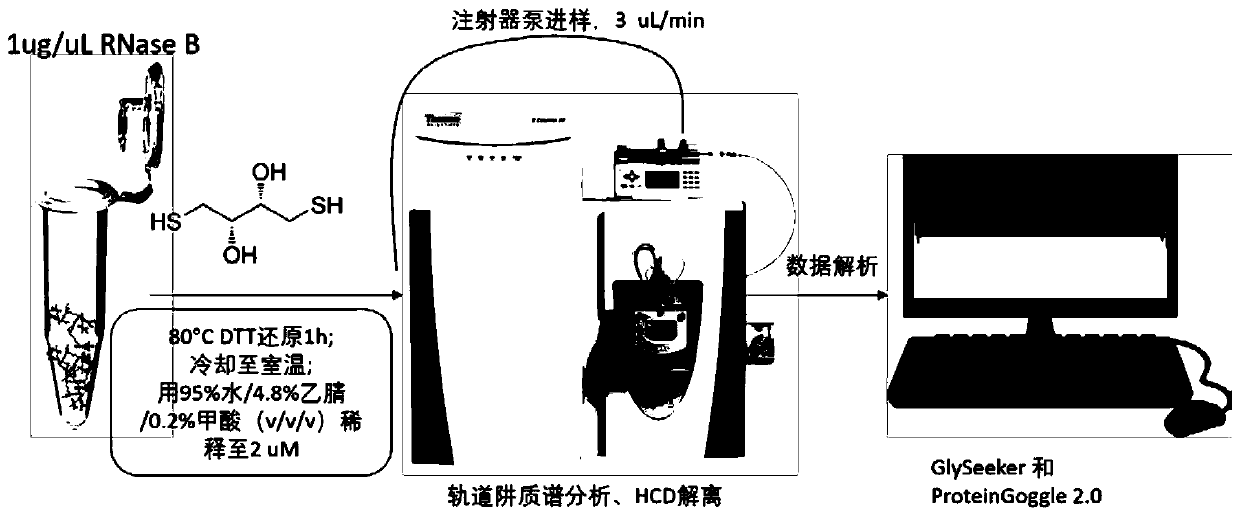
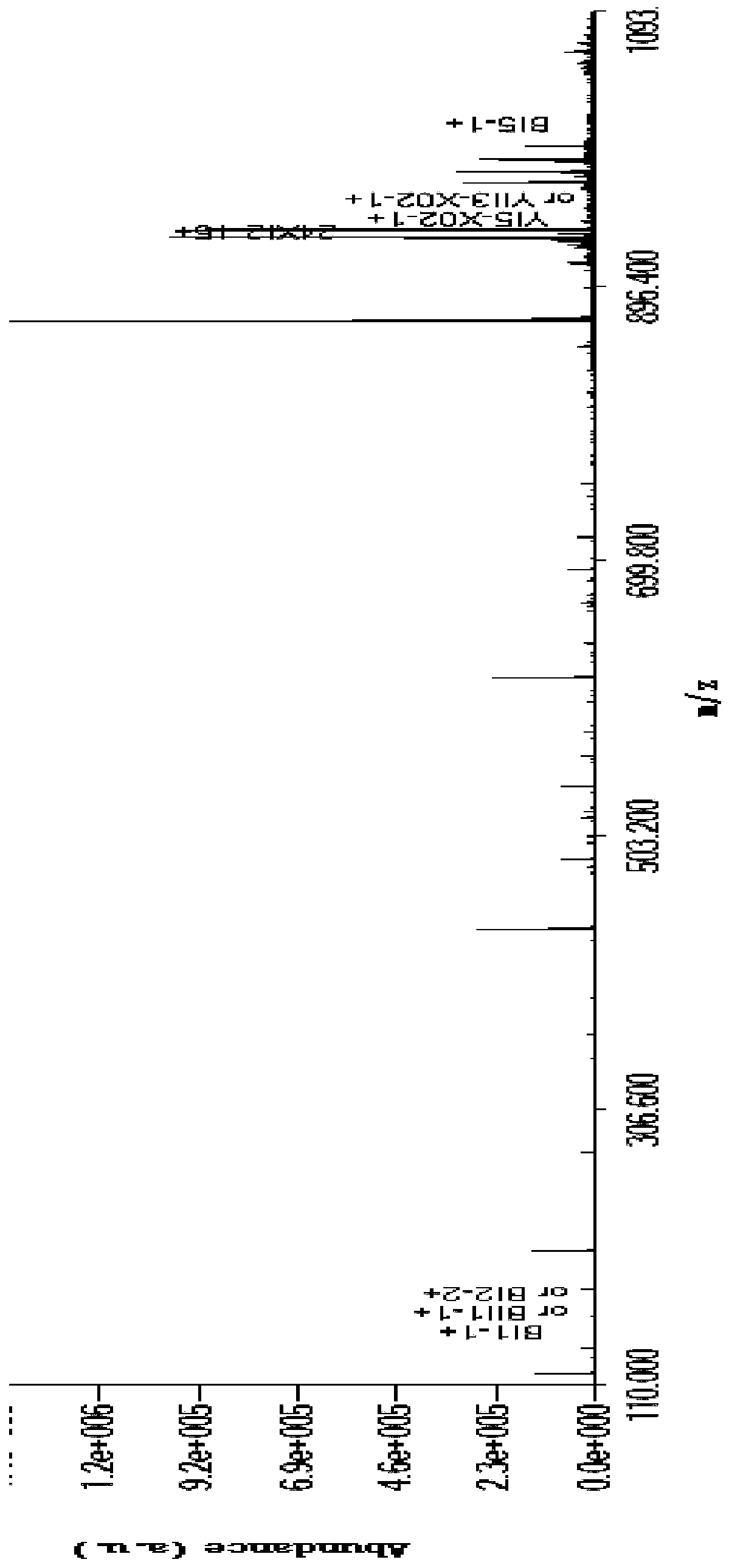
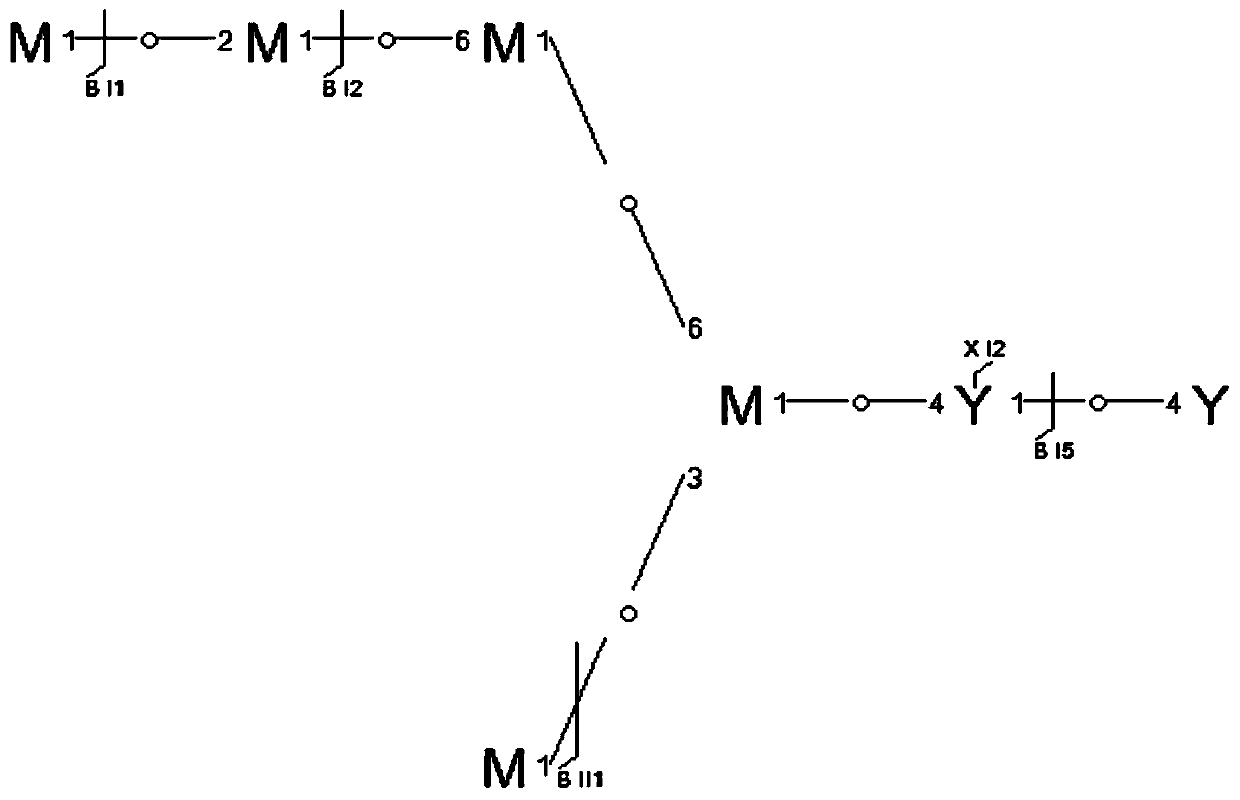
[0091] Characterization of intact N-glycoprotein RNase B
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a method for completely identifying a primary structure of complete N-glycoproteins. The method comprises the following steps: sample pretreatment of N-glycoproteins., namelyreacting a glycoprotein solution and a DTT (Dithiothreitol) solution, and diluting with an electrospray buffer solution; loading samples by a syringe and a syringe pump, spraying in a positive ion mode, and collecting a primary spectrum and a secondary spectrum of the samples by an orbitrap mass spectrometer based on HCD (Host Controller Driver) dissociation; generating an N-glycoprotein precursorion theory database; generating an N-polysaccharide precursor ion theory database; analyzing an N-polysaccharide topological structure; analyzing the N-glycoproteins structure. Compared with the prior art, the method disclosed by the invention has the advantages that the polysaccharide topological structure and the protein structure can be simultaneously detected on a complete protein level, andselectively dissociated characteristic fragment ions of the polysaccharides are obtained. The method is simple in step and high-efficiency and accurate in identification. The method is applicable to polysaccharide topological structure identification, glycosylation site determination and protein scaffold analysis of glycoproteins based on high-resolution tandem mass spectrometry.
Description
technical field [0001] The present invention relates to technical fields such as systems biology and glycoproteomics related to biological mass spectrometry, and in particular relates to a method. Background technique [0002] Glycosylation is one of the most common and important post-translational modifications on proteins. More than 50% of proteins in the human body are glycosylated, and glycosylation has extremely important physiological and pathological functions (Rudd, P.M.; Elliott, T.; Cresswell, P.; Wilson, I.A.; Dwek, R.A., Glycosylation and the immune system. Science 2001, 291(5512), 2370-2376.). Glycoproteomics based on tandem mass spectrometry has become one of the important analytical methods for the identification and analysis of protein amino acid sequence, protein glycosylation site, monosaccharide composition and topological structure ((a) Raman, R.; Raguram, S .; Venkataraman, G.; Paulson, J.C.; Sasisekharan, R., Glycomics: an integrated systems approach t...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): G01N33/68
CPCG01N33/6842G01N33/6848G01N2333/47G01N2560/00G01N2570/00
Inventor 李莎莎田志新
Owner TONGJI UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com