Chemiluminescence immunoassay assay determination kit for detecting human growth hormone and preparation method thereof
A chemiluminescence immunoassay and human growth hormone technology, applied in the field of immunoassay, can solve the problems of poor repeatability, long detection time, low sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
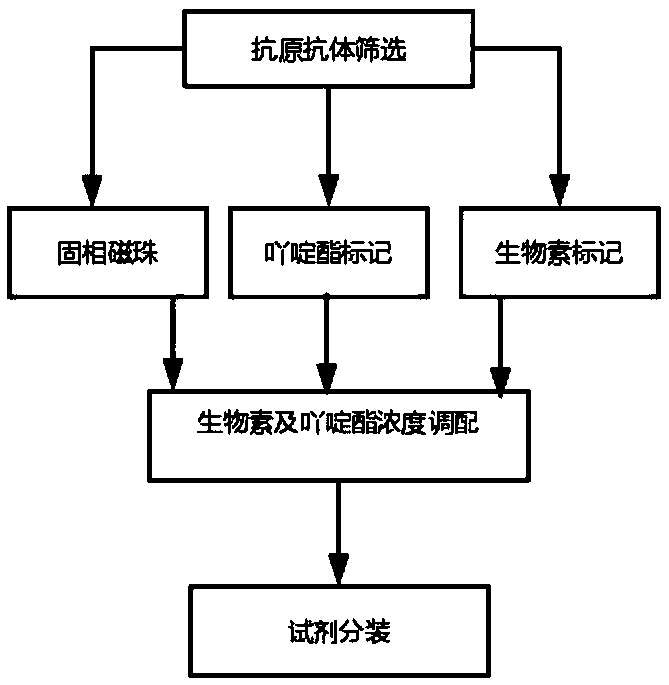
[0041] The present invention also includes a preparation method for detecting human growth hormone chemiluminescence immunoassay assay kit, the specific process is as follows: figure 1 shown, including:
[0042] Step 1: Preparation of magnetic bead suspension coated with streptavidin
[0043] After mixing the streptavidin magnetic particle solution and TBST solution, place it on a magnetic separator until the supernatant is free of turbidity, discard the supernatant, keep the magnetic particles, and prepare a solid phase reagent in the buffer after washing R1; the concentration of the streptavidin magnetic particle solution is preferably 100mg / ml; the volume ratio of the streptavidin magnetic particle solution and TBST solution is preferably 0.5:10; the buffer is 50mM MES, 0.05% temperature, 0.05% Proclin300, pH6.0; the concentration of the magnetic beads coated with streptavidin in the solid-phase reagent is preferably 0.05%-0.1%;
[0044] Step 2: Preparation of acridinium ...
Embodiment 1
[0053] Embodiment 1 prepares GH chemiluminescent detection kit of the present invention
[0054] 1. Preparation of calibrator
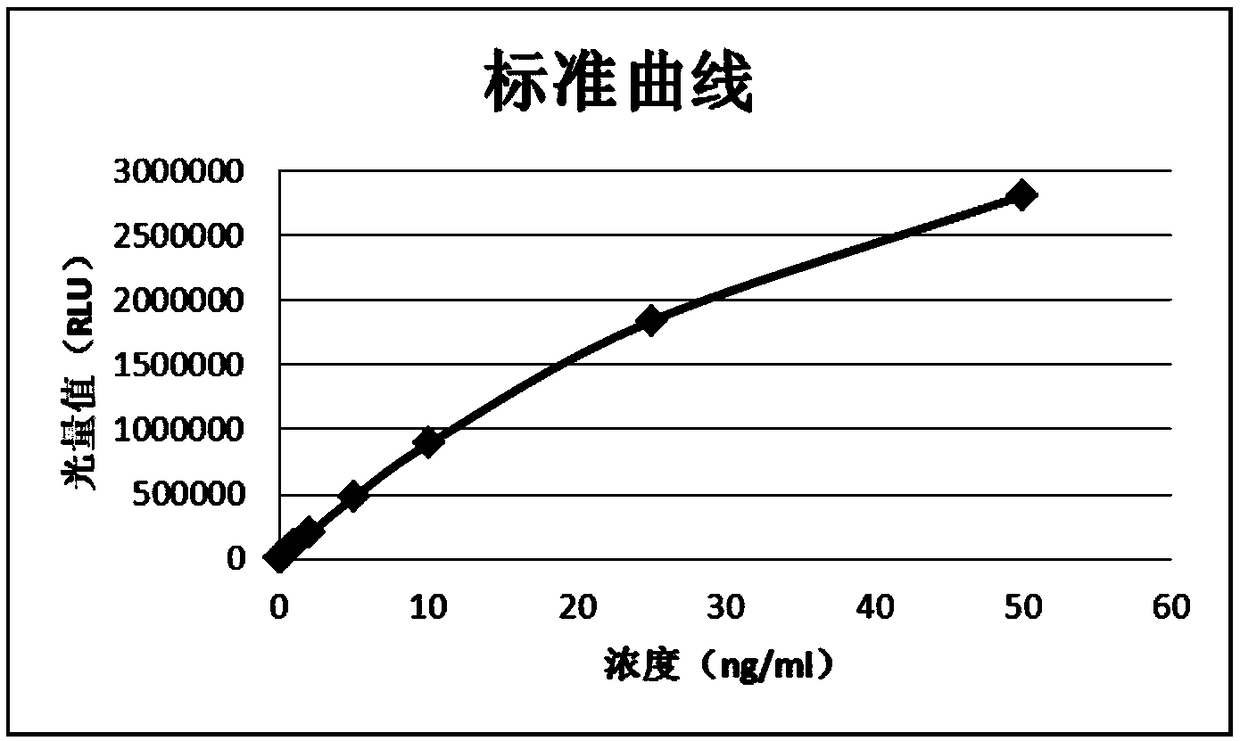
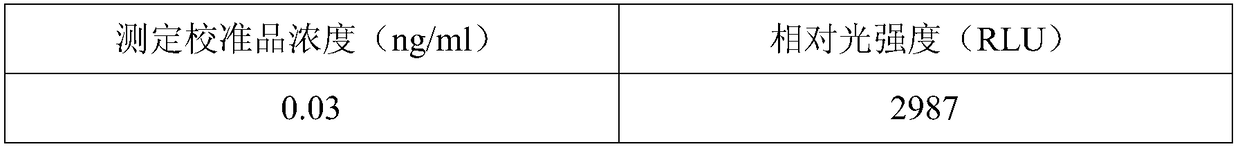
[0055] Dilute GH into a calibrator with 100mM PBS buffer containing 20% calf serum, aliquot into 0.03ng / ml, 0.1ng / ml, 0.5ng / ml, 1ng / ml, 2ng / ml, 5ng / ml, 10ng / ml, 25ng / ml, 50ng / ml.
[0056] 2. Preparation of R1 solution coated with streptavidin magnetic beads
[0057] Take 0.5 ml (50 mg) of streptavidin magnetic particle solution with a concentration of 100 mg / ml, add 10 ml of TBST solution and mix well for 10 minutes, then place it on a magnetic separator until the supernatant is clear of turbidity, discard the supernatant , to retain the magnetic particles. After repeated washing for 3 times, a solid-phase reagent with a magnetic bead concentration of 0.05% was formulated in a buffer solution of 50mM MES, 0.05% Tween, 0.05% Proclin300, pH 6.0, and stored at 2-8°C.
[0058] 3. Preparation of acridinium ester-labeled GH monoclonal antibody R2 sol...
Embodiment 2
[0062] Embodiment 2 Preparation of GH chemiluminescence detection kit of the present invention
[0063] 1. Preparation of calibrator
[0064] Dilute GH into a calibrator with 100mM PBS buffer containing 20% calf serum, aliquot into 0.03ng / ml, 0.1ng / ml, 0.5ng / ml, 1ng / ml, 2ng / ml, 5ng / ml, 10ng / ml, 25ng / ml, 50ng / ml.
[0065] 2. Preparation of R1 solution coated with streptavidin magnetic beads
[0066] Take 0.5 ml (50 mg) of streptavidin magnetic particle solution with a concentration of 100 mg / ml, add 10 ml of TBST solution and mix well for 10 minutes, then place it on a magnetic separator until the supernatant is clear of turbidity, discard the supernatant , to retain the magnetic particles. After repeated washing for 3 times, a solid-phase reagent with a magnetic bead concentration of 0.03% was formulated in a buffer solution of 50mM MES, 0.05% Tween, 0.05% Proclin300, pH 6.0, and stored at 2-8°C.
[0067] 3. Preparation of acridinium ester-labeled GH monoclonal antibody...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com