Oncofetal fibronectin detection method using simple immunoassay
A fibronectin and carcinoembryonic technology, applied in the field of detection of carcinoembryonic fibronectin using immunochromatography, can solve the problems of not describing the similarity of antigens, etc., and achieve the effect of reducing cross-reactivity, preventing measurement errors or false positive reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
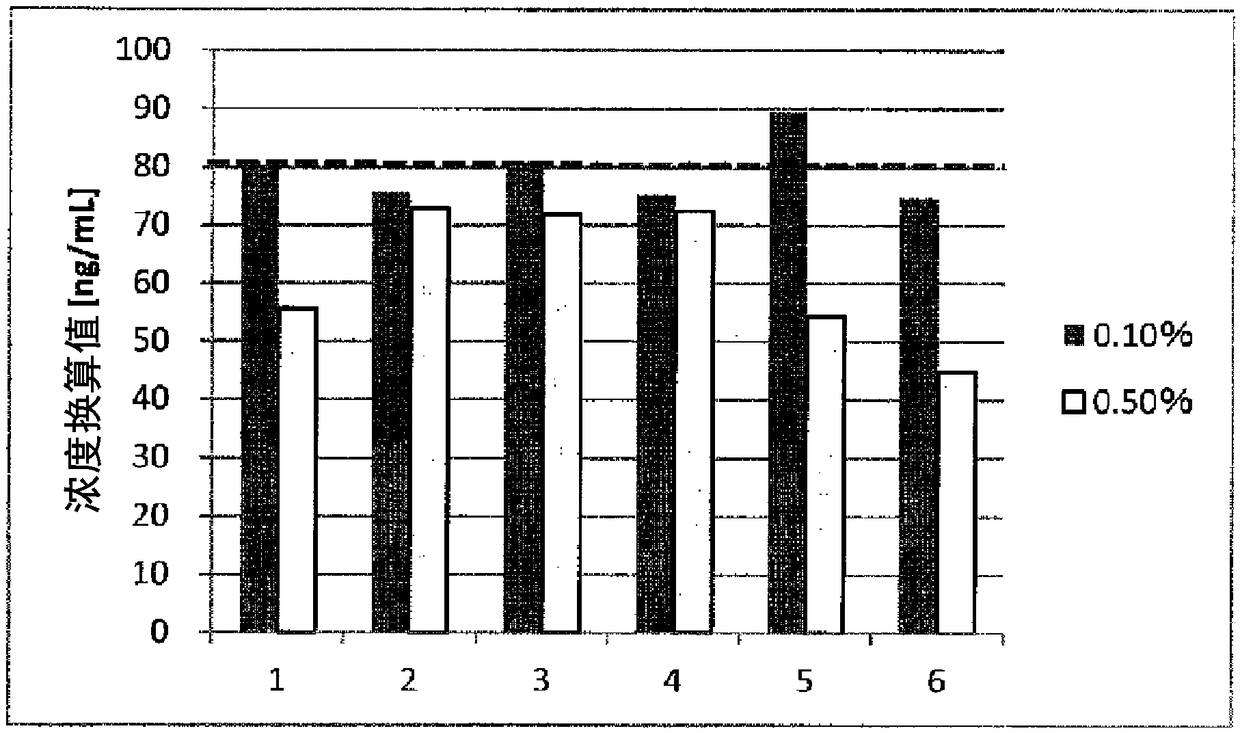
[0183] [Example 1] Relationship between the type of surfactant added to the sample extract and FN cross-reactivity
[0184] The type of surfactant added to the sample extract was changed by using the detection device fabricated above, and its influence on the cross-reactivity of plasma FN was confirmed. For concentrations of nonionic surfactants, % means % (w / v) unless otherwise indicated.
[0185] 1. Test method
[0186] (1) Sample preparation
[0187] The normal human plasma pool was diluted with the fFN sample extract described below to prepare a 0.3% plasma sample.
[0188]
[0189] As shown in Table 1, 10 mM PBS, 1% BSA, 1 mM EDTA-2Na, nonionic surfactant (0.1%, 0.5%).
[0190] [Table 1]
[0191]
1
TritonX-114 (prior art)
2
Triton X-100
3
n-Heptyl-β-D-thioglucoside
4
n-Octyl-β-D-glucoside
5
Tween 20
6
Brij35 (Brij is a trademark)
[0192] (2) Measurement
[0193] 120 ...
Embodiment 2
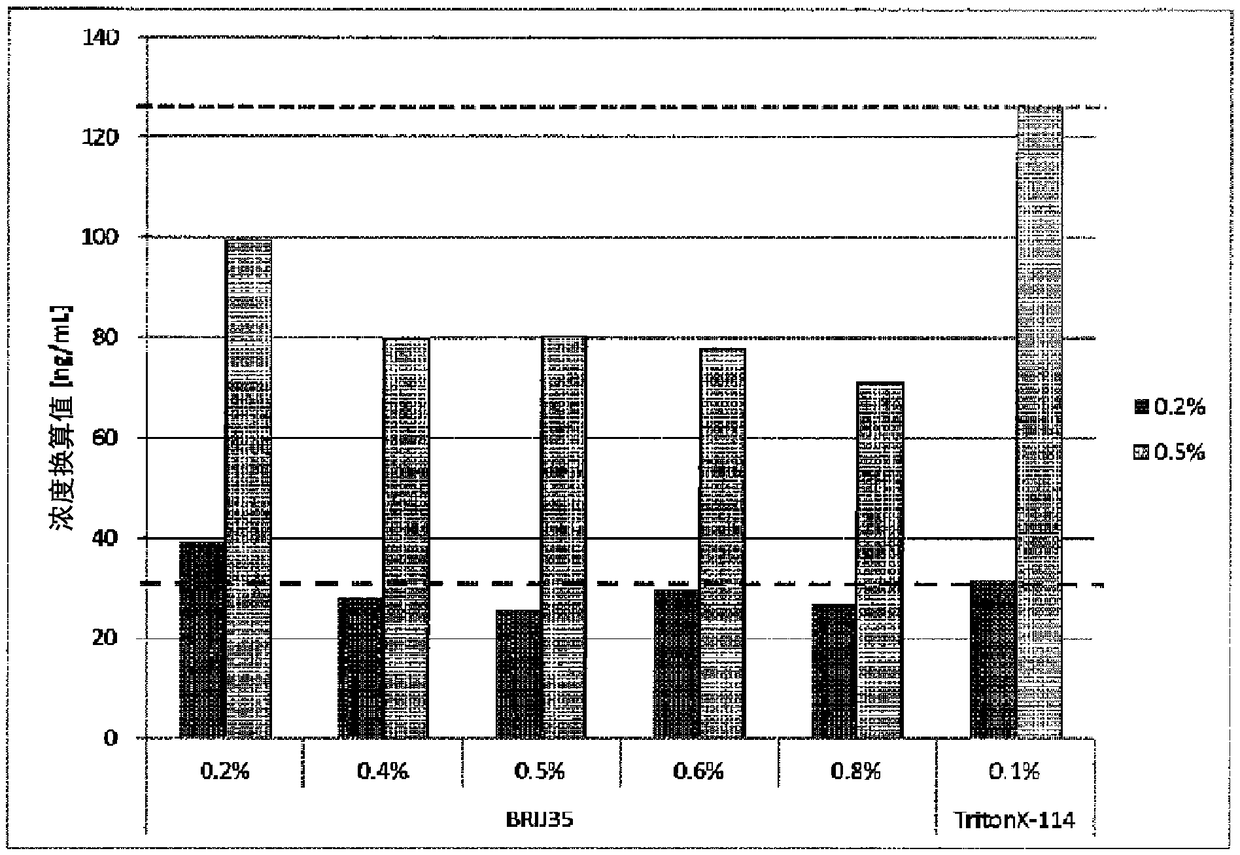
[0197] [Example 2] Relationship between the added concentration of Brij 35 and FN cross-reactivity
[0198] 1. Test method
[0199] Detect in the same manner as in Example 1, except that the normal human plasma confluence (pool) is treated with 0.2%, 0.4%, 0.5%, 0.6% or 0.8% Brij 35 as a nonionic surfactant Dilute the fFN sample extract to adjust the plasma concentration to 0.2% and 0.5%. As a comparative example, the fFN sample extract (prior art) added with Triton X-114 was also tested.
[0200] 2. Test results
[0201] The results are shown in figure 2 middle.
[0202] Compared with the addition of 0.1% Triton X-114 in the prior art, when Brij 35 was added in the concentration range of 0.4% to 0.8%, the cross-reactivity with plasma FN was inhibited.
Embodiment 3
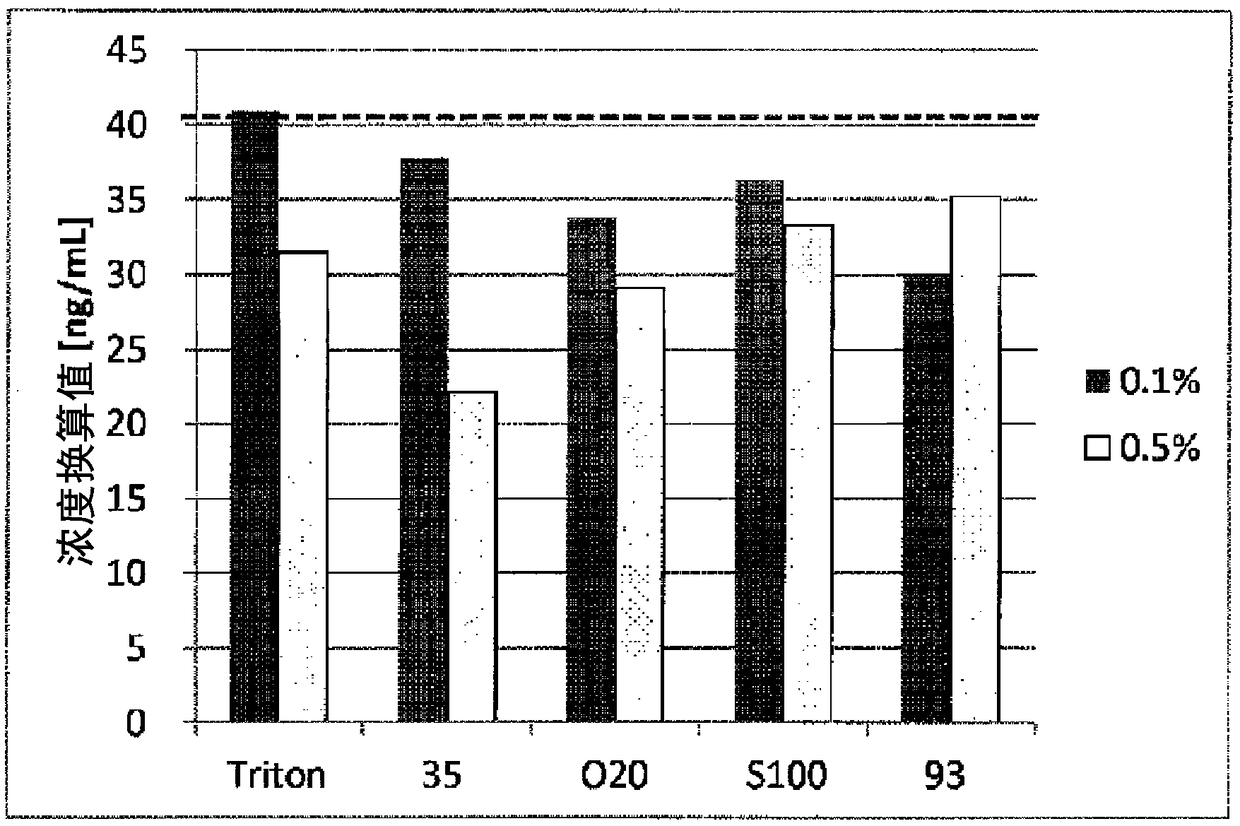
[0203] [Example 3] Relationship between Brij chain length and FN cross-reactivity
[0204] 1. Test method
[0205] Detect in the same manner as in Example 1, except that the plasma confluence of normal people is diluted with the fFN sample extract containing 0.1% or 0.5% of the nonionic surfactants shown in Table 2, to Plasma concentrations were adjusted to 0.2% and 0.3%. As a comparative example, the fFN sample extract (prior art) added with 0.1% or 0.5% Triton X-114 was also tested.
[0206] [Table 2]
[0207]
[0208] 2. Test results
[0209] The results are shown in image 3 middle.
[0210] Both concentrations of Brij 35 (m=23) and Brij O20 (m=20) inhibited the cross-reactivity with plasma fibronectin, thereby exhibiting a high inhibitory effect, compared to the same concentration of Triton X-114. In contrast, 0.5% Brij S100 (m = 100) and Brij 93 (m = 2) could not inhibit the cross-reactivity with plasma fibronectin compared with the same concentration of Triton ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com