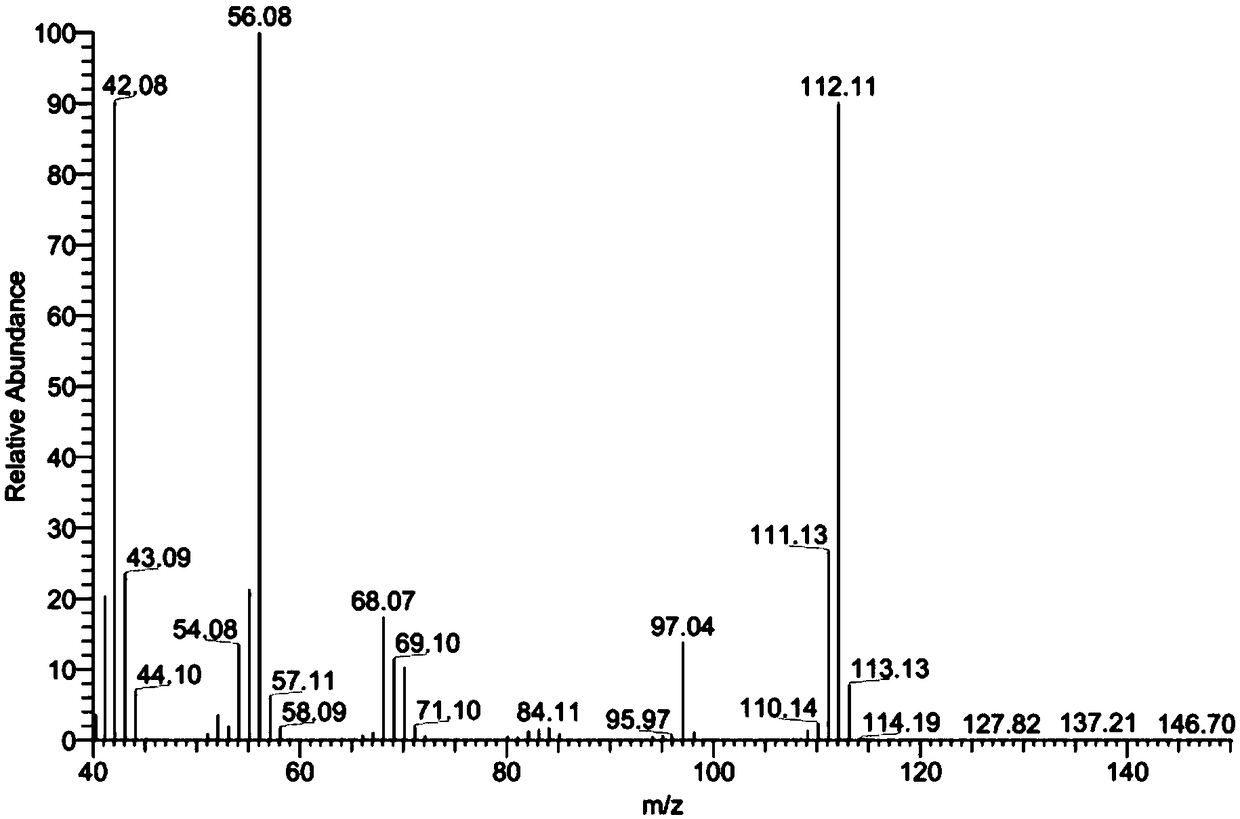
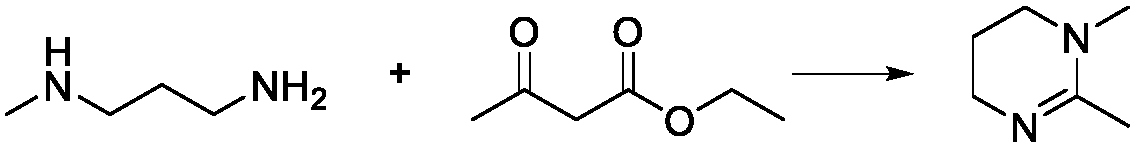
Synthesis method of 1,2-dimethyl-1,4,5,6-tetrahydropyrimidine
A technology of tetrahydropyrimidine and a synthetic method, applied in directions such as organic chemistry, to achieve the effects of high yield, simple reaction route and simple procedure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1, the preparation of N-[3-(methylamino)propyl]-acetamide
[0030] In a 1000mL three-necked flask, add N-methylpropylenediamine (200g, 2.27mol), add dropwise acetyl chloride (182g, 2.31mol) under stirring, control the temperature within 20°C, after the dropwise addition, within 60°C Continue to react for 1h, stop the reaction, and set aside.
[0031] 2. Preparation of 1,2-dimethyl-1,4,5,6-tetrahydropyrimidine
[0032] Add 200mL of PPE to the N-[3-(methylamino)propyl]-acetamide solution obtained in the previous step, control the temperature at 80°C by microwave, and react for 2h. After completion, the product 1,2-di Methyl-1,4,5,6-tetrahydropyrimidine 240g, yield 94.42%.
Embodiment 2
[0034] 1, the preparation of N-[3-(methylamino)propyl]-acetamide
[0035] In a 1000mL three-necked flask, add N-methylpropylenediamine (200g, 2.27mol), add dropwise acetic anhydride (240g, 2.34mol) under stirring, control the temperature within 30°C, and continue the reaction for 1h after the dropwise addition is completed. Stop the reaction and set aside.
[0036] 2. Preparation of 1,2-dimethyl-1,4,5,6-tetrahydropyrimidine
[0037] Add 180mL of PPE to the N-[3-(methylamino)propyl]-acetamide solution obtained in the previous step, control the temperature by microwave at 70°C, and react for 1.5h. After completion, the product 1,2- Dimethyl-1,4,5,6-tetrahydropyrimidine 230g, yield 90.49%.
Embodiment 3
[0039] 1, the preparation of N-[3-(methylamino)propyl]-acetamide
[0040] In a 1000mL three-necked flask, add N-methylpropylenediamine (200g, 2.27mol), add acetic anhydride (2.49mol) dropwise under stirring, control the temperature within 25°C, after the dropwise addition is completed, continue the reaction for 1h and stop the reaction ,spare.
[0041] 2. Preparation of 1,2-dimethyl-1,4,5,6-tetrahydropyrimidine
[0042] Add 180mL of PPE to the N-[3-(methylamino)propyl]-acetamide solution obtained in the previous step, control the temperature at 60°C by microwave, and react for 1.8h. After completion, the product 1,2- Dimethyl-1,4,5,6-tetrahydropyrimidine 235g, yield 92.45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com