Medical clinical research and development information processing system and method thereof
An information processing system and medical technology, which is applied in the field of information processing of medical research and development, can solve the problems of syndromes and non-biochemical inspection indicators, clinical response information distortion, and inability to obtain timely, complete and effective records, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
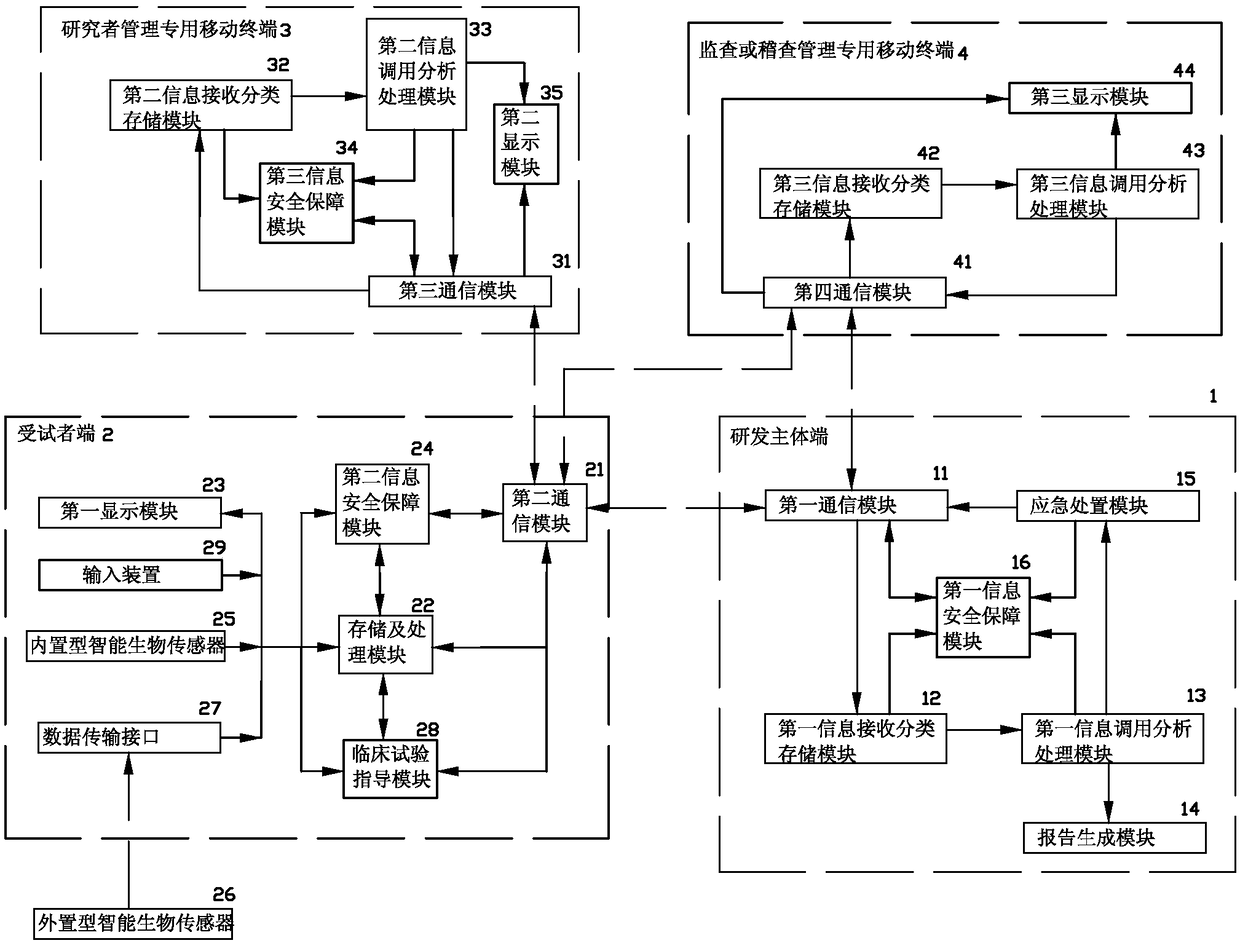
[0035] see figure 1 As shown, a kind of medicine clinical research and development information processing system of the present invention comprises:
[0036] R&D subject terminal 1, which has a first communication module 11, a first information receiving and classification storage module 12, a first information call analysis and processing module 13, a report generation module 14, an emergency handling module 15 and a first information security guarantee module 16; Wherein, the first communication module 11 is respectively connected with the first information receiving classification storage module 12, the emergency treatment module 15 and the first information security guarantee module 16, and the first information calling analysis processing module 13 is respectively connected with the first information receiving classification storage module 12. The report generating module 14, the emergency handling module 15 and the first information security guarantee module 16 are conne...
Embodiment 2
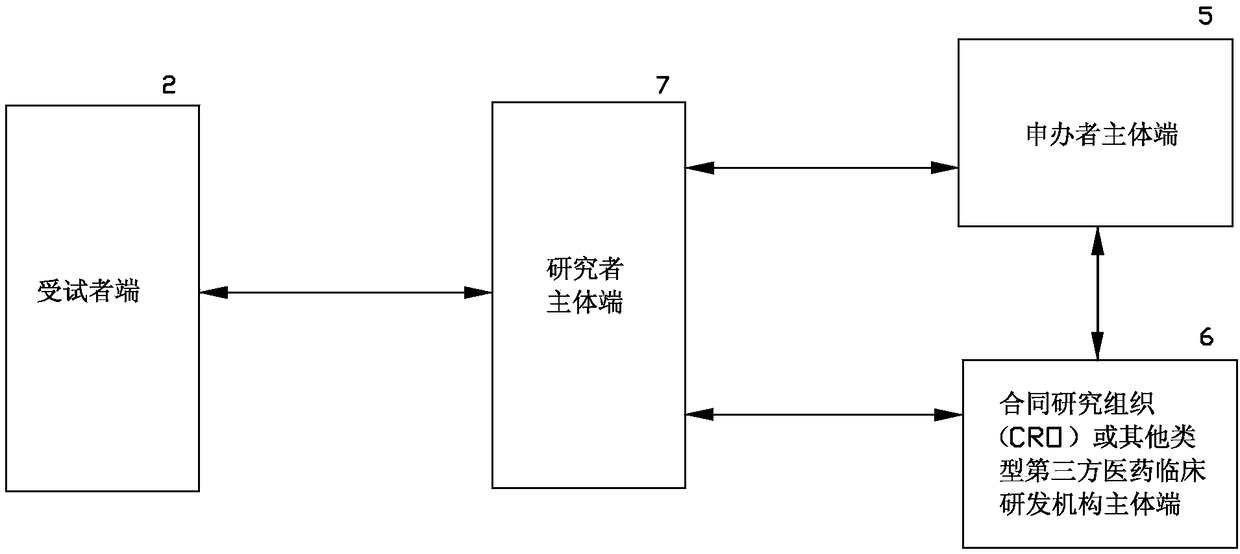
[0061] see figure 2 As shown, a medical clinical research and development information processing system and its method of the present invention are different from Embodiment 1 in that the research and development main body is further extended and expanded into Sponsor main terminal 5, contract research organization (CRO) or other types of third-party pharmaceutical clinical research and development institutions main terminal 6, researcher main terminal 7; in this embodiment, researcher main terminal 7 communicates with the subject terminal through the Internet 2 Realize information interaction, the sponsor main terminal 5 and the main terminal 6 of the contract research organization (CRO) or other types of third-party pharmaceutical clinical research and development institutions realize information interaction with the subject terminal 2 via the researcher main terminal 7, Information exchange between the main body 5 of the sponsor and the main body 6 of the contract research...
Embodiment 3
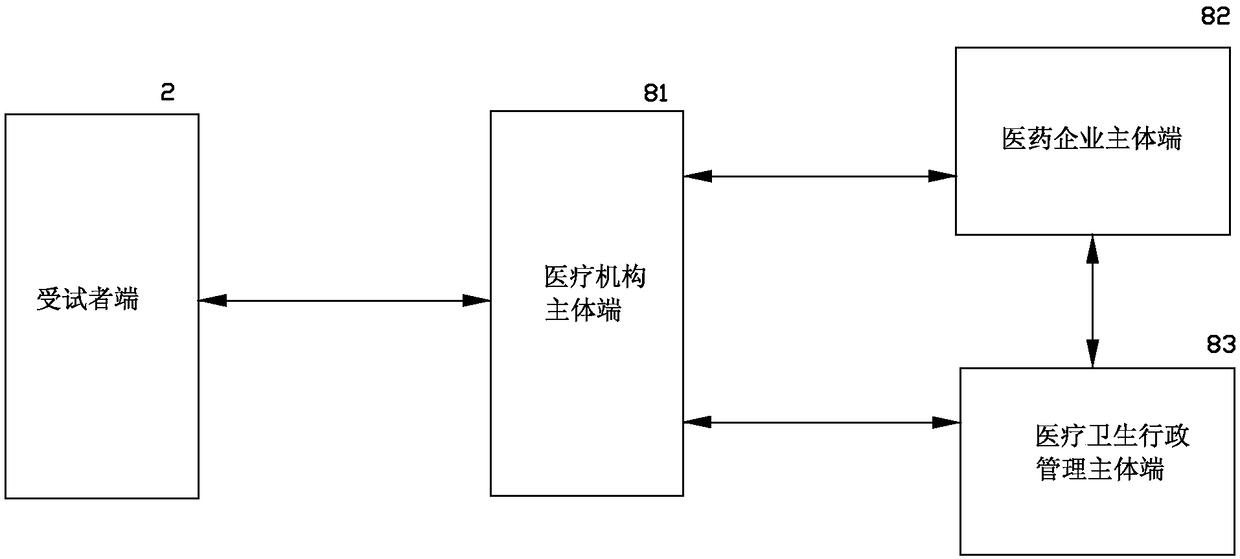
[0063] see image 3 As shown, a medical clinical research and development information processing system and its method of the present invention are different from Embodiment 1 in that the research and development subject end is further extended into a medical institution main end 81, a pharmaceutical enterprise main end 82, One or more of the main terminal 83 of medical and health administrative management; and the medical and health administration main terminal 83 to realize information interaction between the medical institution main terminal 81 and the subject terminal 2; of course, it can also be the medical institution main terminal 81, the pharmaceutical enterprise main The administrative management subject terminal 83 realizes information interaction directly with the subject terminal 2 respectively through the Internet, and the medical institution main terminal 81, the pharmaceutical enterprise main terminal 82, and the medical and health administrative management subj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com