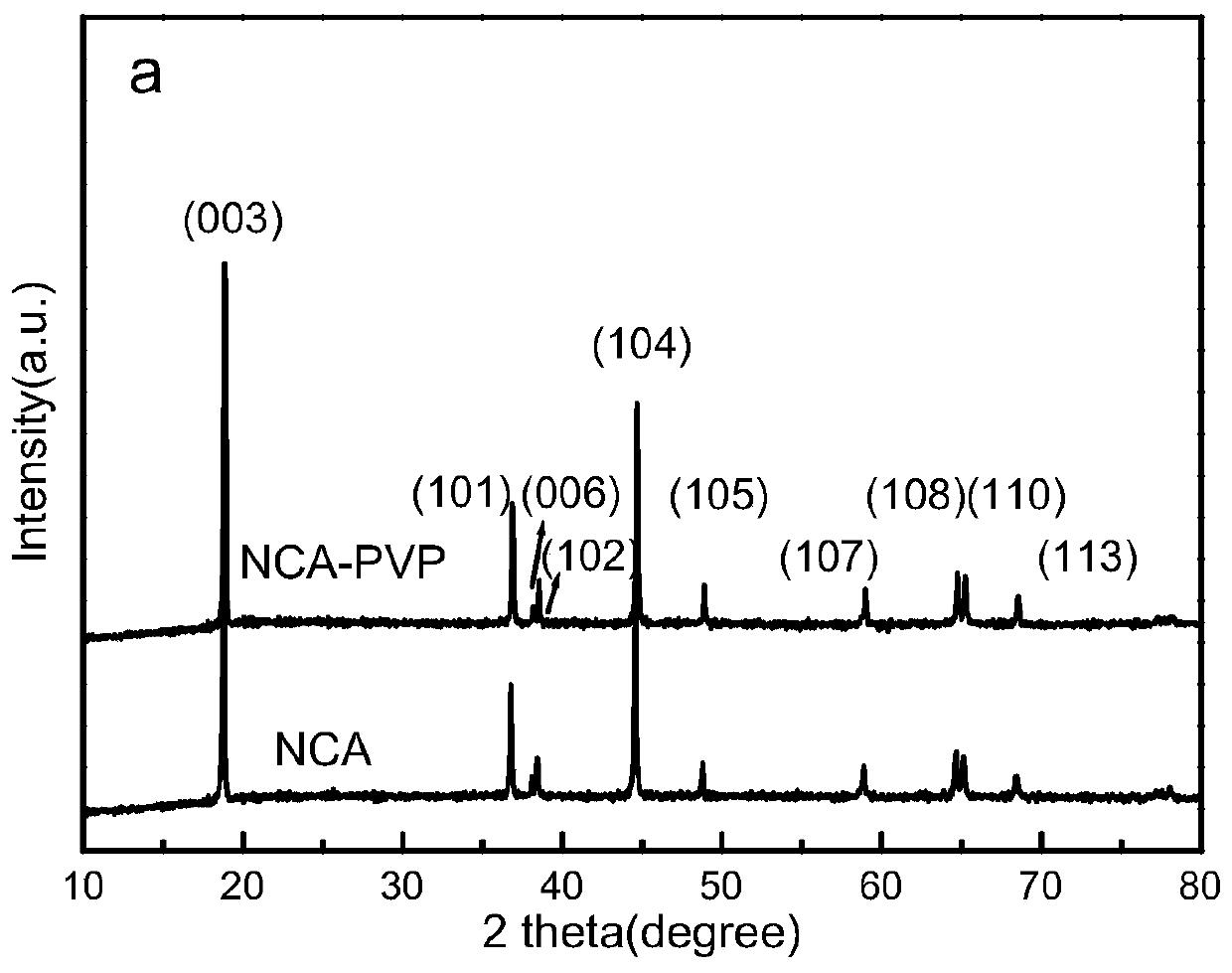
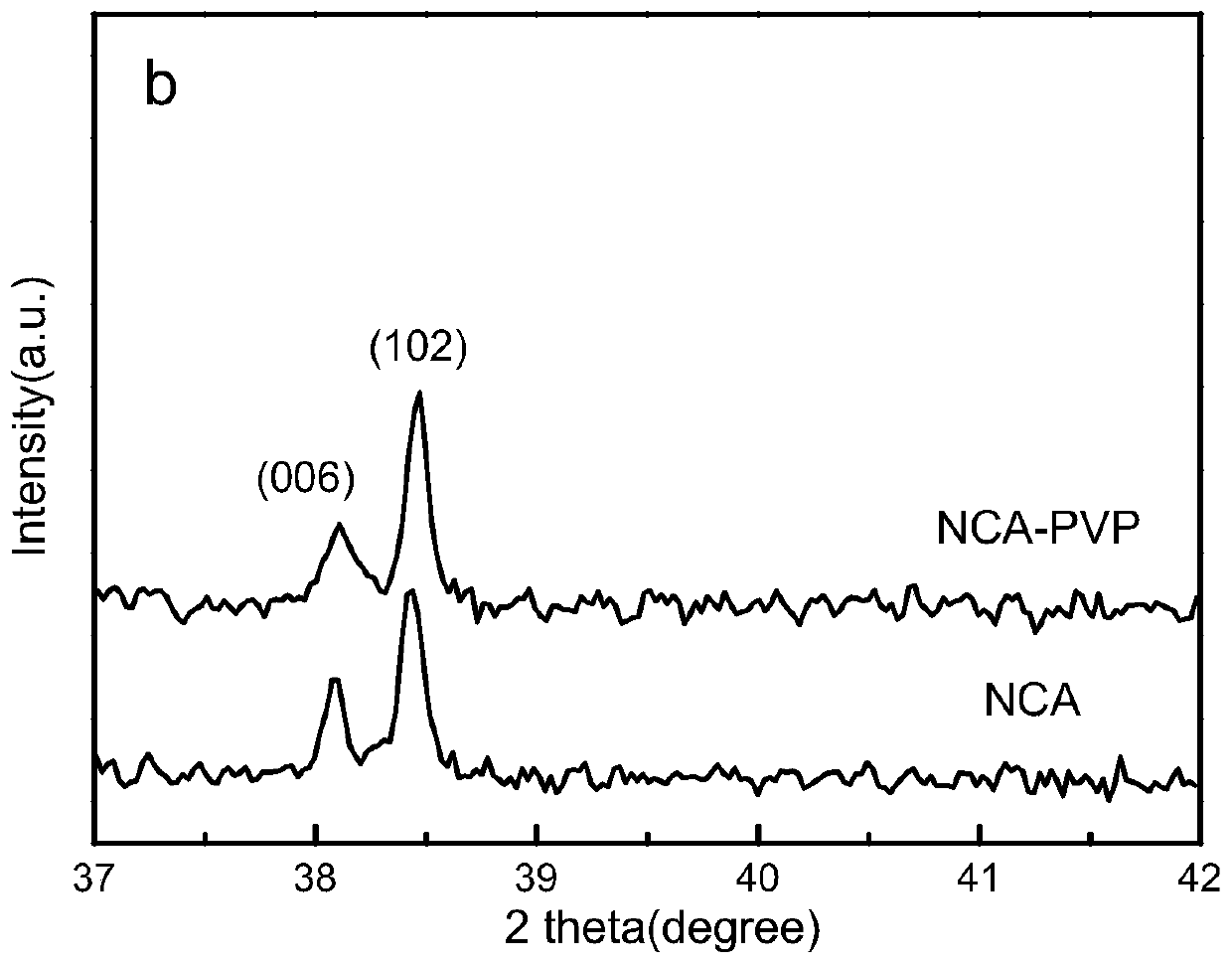
lini 0.8 co 0.15 al 0.05 o 2 Positive electrode material and preparation method thereof
A positive electrode material, cobalt salt technology, applied in the field of lithium batteries, can solve the problems of increased irreversible capacity, low initial charge and discharge efficiency, and poor rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of LiNi that present embodiment provides 0.8 co 0.15 Al 0.05 o 2 The positive electrode material is prepared as follows:
[0045] Weigh 0.5mmol CoSO 4 ·7H 2 O was dissolved in 25ml deionized water, and the labeled solution was A. Weigh 24mmolNiSO 4 ·6H 2 O, 4.0mmol CoSO 4 ·7H 2 O, 0.75mmol Al 2 (SO 4 ) 3 18H 2 O was dissolved in 75ml deionized water, and the labeled solution was B. Weigh 36mmol H 2 C 2 o 4 2H 2 Dissolve O in 120ml deionized water, add 0.1324g PVP while stirring, and mark the solution as C. With continuous stirring, solution A was slowly added dropwise to solution C to obtain a suspension. Then solution B was added dropwise to the suspension at a certain speed. After the dropwise addition was completed, it was stirred for 2 hours, then filtered with suction, and the precipitate was washed, and finally vacuum-dried at 80°C for 12 hours. The obtained sample is the precursor of cathode material, marked as MC 2 o 4 2H 2 O-PVP. ...
Embodiment 2
[0047] A kind of LiNi that present embodiment provides 0.8 co 0.15 Al 0.05 o 2 The positive electrode material is prepared as follows:
[0048] Weigh 0.5mmol CoSO 4 ·7H 2 O was dissolved in 25ml deionized water, and the labeled solution was A. Weigh 24mmolNiSO 4 ·6H 2 O, 4.0mmol CoSO 4 ·7H 2 O, 0.75mmol Al 2 (SO 4 ) 3 18H 2 O was dissolved in 75ml deionized water, and the labeled solution was B. Weigh 36mmol H 2 C 2 o 4 2H 2 Dissolve O in 120ml of deionized water, add 0.662g of PVP while stirring, and mark the solution as C. With continuous stirring, solution A was slowly added dropwise to solution C to obtain a suspension. Then, solution B was added dropwise to the suspension at a certain speed. After the dropwise addition was completed, it was stirred for 3 hours, then suction filtered, and the precipitate was washed, and finally vacuum-dried at 75°C for 10 hours. The obtained sample is the precursor of cathode material, marked as MC 2 o 4 2H 2 O-PVP. ...
Embodiment 3
[0050] A kind of LiNi that present embodiment provides 0.8 co 0.15 Al 0.05 o 2 The positive electrode material is prepared as follows:
[0051] Weigh 0.5mmol CoSO 4 ·7H 2 O was dissolved in 25ml deionized water, and the labeled solution was A. Weigh 24mmolNiSO 4 ·6H 2 O, 4.0mmol CoSO 4 ·7H 2 O, 0.75mmol Al 2 (SO 4 ) 3 18H 2 O was dissolved in 75ml deionized water, and the labeled solution was B. Weigh 36mmol H 2 C 2 o 4 2H 2 Dissolve O in 120ml of deionized water, add 1.986g of PVP while stirring, and mark the solution as C. With continuous stirring, solution A was slowly added dropwise to solution C to obtain a suspension. Then, solution B was added dropwise to the suspension at a certain speed. After the dropwise addition was completed, it was stirred for 1 hour, then filtered with suction, and the precipitate was washed, and finally vacuum-dried at 85°C for 15 hours. The obtained sample is the precursor of cathode material, marked as MC 2 o 4 2H 2 O-P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com