Benzimidazole-containing Tr*ger's base type compound as well as preparation method and application thereof
A benzimidazole and compound technology, which is applied in the field of drug synthesis, can solve the problems of limited number of chemotherapeutic drugs, cell damage, toxic and side effects, and achieves the effects of low cytotoxicity, mild reaction conditions and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
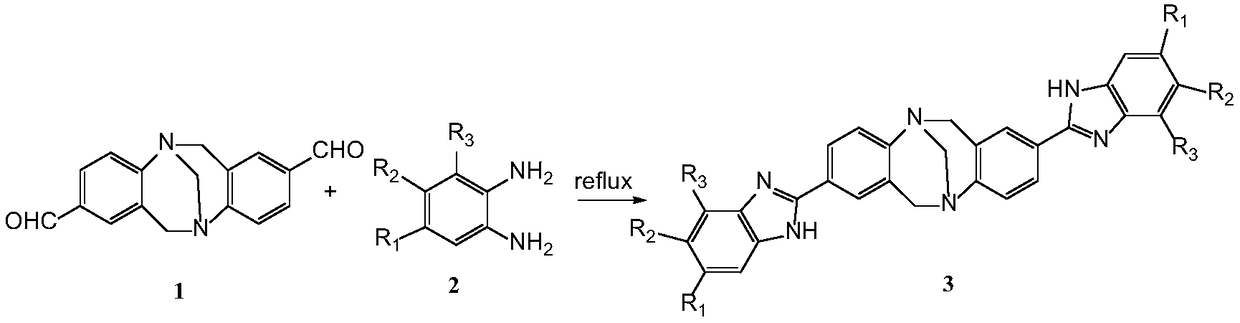
[0030] Add 0.5mmol 2,8-dialdehyde-TB, 1.2mmol 4-methyl-o-phenylenediamine and 20mL 1,4-dioxane into a 50mL dry eggplant-shaped bottle, and react under reflux at 102°C for 4 hours. After cooling to room temperature, the excess solvent was evaporated under reduced pressure, and the crude product was separated by column chromatography, using methanol: ethyl acetate = 1:8 (V / V) as the eluent, and the product 3a was isolated and purified (yield: 87% ).
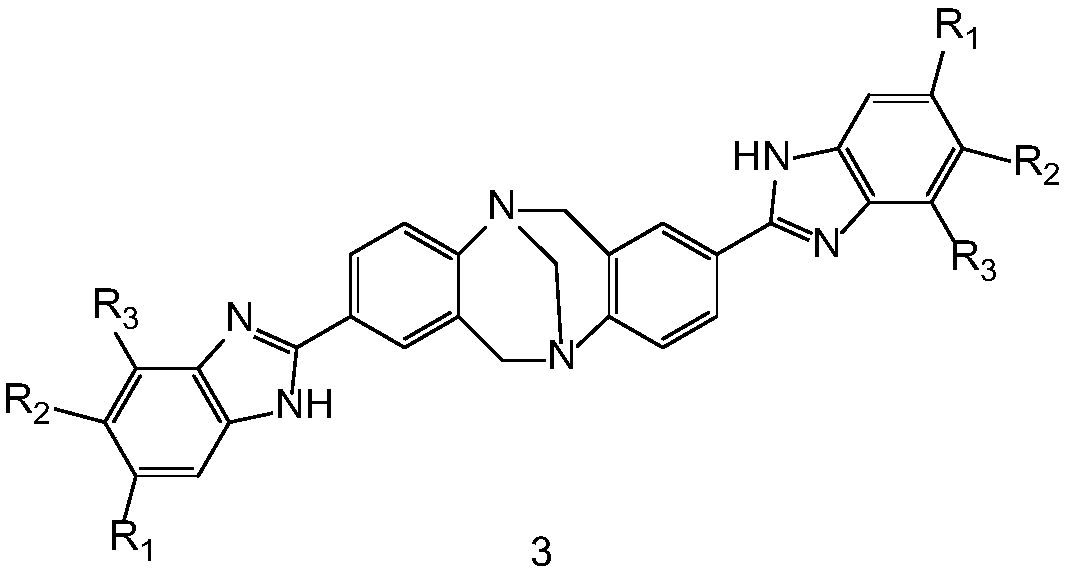
[0031] The structural formula of compound 3a is:
[0032]
[0033] The molecular formula is: C 31 h 27 N 6
[0034] Chinese name: 2,8-bis(5-methyl-1H-benzo[d]imidazol-2-yl)-6H,12H-5,11-methyldibenzo[b,f][1, 5] Diazocine
[0035] English name: 2,8-bis(5-methyl-1H-benzo[d]imidazol-2-yl)-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine
[0036] Appearance: black solid
[0037] Melting point: 257.2-259.2°C
[0038] Proton NMR spectrum: 1 H NMR (400MHz, DMSO-d 6 )δ12.60(s,2H),7.93(d,J=7.8Hz,2H),7.79(s,2H),7.41(s,2H),7.32(d,J=...
Embodiment 2
[0042] Add 0.5mmol 2,8-dialdehyde-TB, 1.2mmol 4-methoxy-o-phenylenediamine, and 20mL 1,4-dioxane to a 50mL dry eggplant-shaped bottle, reflux and stir at 102°C for 4 hours, and the reaction is complete After cooling to room temperature, the excess solvent was evaporated under reduced pressure, and the crude product was separated by column chromatography, using methanol:ethyl acetate=1:8 (V / V) as the eluent, and the product 3b was isolated and purified (yield: 81 %).
[0043] The structural formula of compound 3b is:
[0044] The molecular formula is: C 31 h 27 N 6 o 2
[0045] Chinese name: 2,8-bis(5-methoxy-1H-benzo[d]imidazol-2-yl)-6H,12H-5,11-methylenedibenzo[b,f][ 1,5] diazocine
[0046] English name: 2,8-bis(5-methoxy-1H-benzo[d]imidazol-2-yl)-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine
[0047] Appearance: dark gray solid
[0048] Melting point: 234.6-236.4°C
[0049] Proton NMR spectrum: 1 H NMR (400MHz, DMSO-d 6 )δ12.59(s,2H),7.90(s,2H),7.76(s,2H),7.46(s,...
Embodiment 3
[0053] Add 0.5mmol 2,8-dialdehyde-TB, 1.2mmol 4,5-dichloro-o-phenylenediamine and 20mL 1,4-dioxane into a 50mL dry eggplant-shaped bottle, reflux and stir at 102°C for 4h, After the reaction was completed, it was cooled to room temperature, and the excess solvent was evaporated under reduced pressure. The crude product was separated by column chromatography, using methanol:ethyl acetate=1:8 (V / V) as the eluent, and the product 3c was isolated and purified (yield : 89%).
[0054] The structural formula of compound 3c is:
[0055] The molecular formula is: C 29 h 19 Cl 4 N 6
[0056] Chinese name: 2,8-bis(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-6H,12H-5,11-methylenedibenzo[b,f] [1,5]Diazocine
[0057] English name: 2,8-bis(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine
[0058] Appearance: light gray solid
[0059] Melting point: >300°C
[0060] Proton NMR spectrum: 1 H NMR (400MHz, DMSO-d 6 )δ13.12(s,2H),7.95(d,J=8.0Hz,2H),7.84...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com