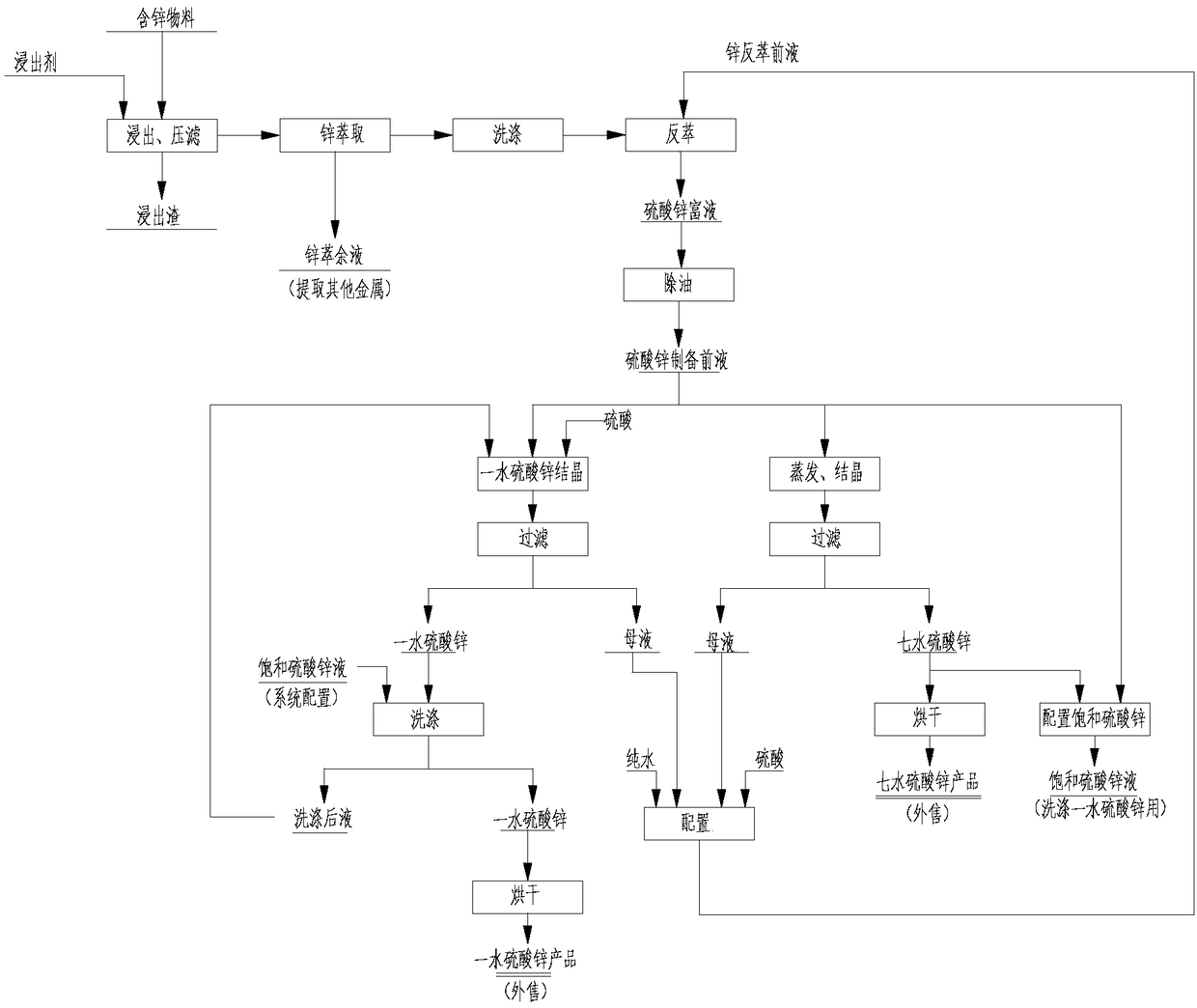
Method for joint production of zinc sulphate monohydrate and zinc sulphate heptahydrate
A joint production technology of zinc sulfate heptahydrate, applied in the direction of zinc sulfate, etc., can solve the problems of tediousness, high production cost, large investment in equipment, etc., and achieve the effect of reducing costs and improving competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The material containing 40% zinc is leached and filtered with hydrochloric acid, and the pH of the leaching solution is controlled at 3.0-5.0 to obtain a zinc chloride leaching solution. The leaching solution is mixed and extracted with P204 extractant to obtain an organic phase loaded with zinc ions. The organic phase is passed through After washing, use 1.5-4mol / L H 2 SO 4 The back-extraction solution (the mother liquor obtained after the follow-up process produces zinc sulfate monohydrate and zinc sulfate heptahydrate, and the pre-extraction solution obtained after adding sulfuric acid and water for configuration is returned and used as a back-extraction solution) to obtain zinc-containing in 180g / L zinc sulfate rich solution. The zinc sulfate-rich solution is degreased by activated carbon to obtain a pre-preparation solution of zinc sulfate with an oil content of less than 10 ppm. Prepare the pre-liquid of zinc sulfate, pump a part of it into the crystallization t...
Embodiment 2
[0047]The material containing 25% zinc is leached and filtered by sulfuric acid, and the pH of the leachate is controlled at 3.0-5.0 to obtain a zinc sulfate leachate, which is mixed and extracted with P204 extractant to obtain an organic phase loaded with zinc ions. The organic phase is washed After that, use 1.5-4mol / L H 2 SO 4 The back-extraction solution (the mother liquor obtained after the follow-up process produces zinc sulfate monohydrate and zinc sulfate heptahydrate, and the pre-extraction solution obtained after adding sulfuric acid and water for configuration is returned and used as a back-extraction solution) for back-extraction to obtain zinc-containing in 200g / L zinc sulfate rich solution. The zinc sulfate-rich solution is degreased by activated carbon to obtain a pre-preparation solution of zinc sulfate with an oil content of less than 10 ppm. Prepare a part of the zinc sulfate to prepare the pre-liquid, pump it into the crystallization tank, start stirring, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com