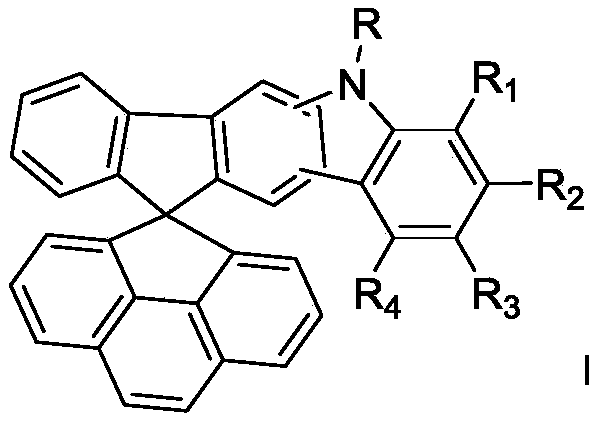
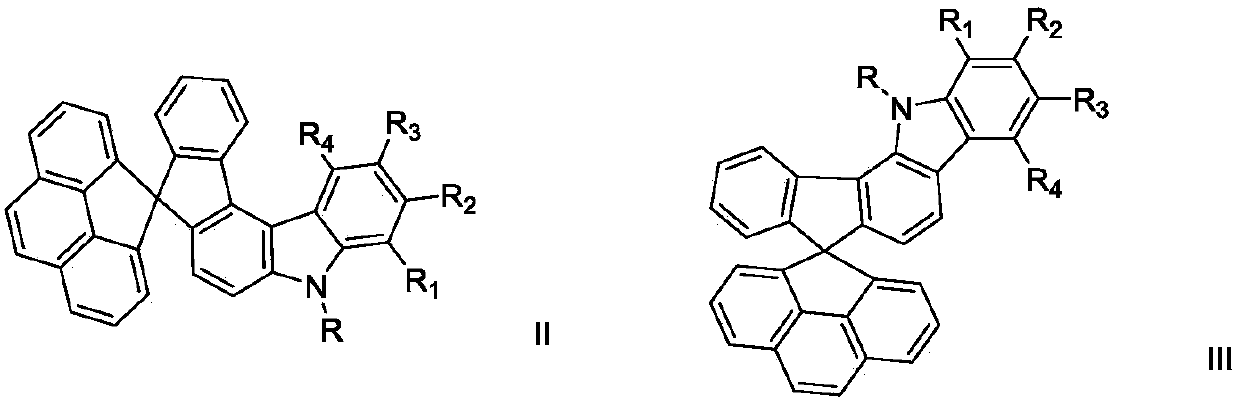
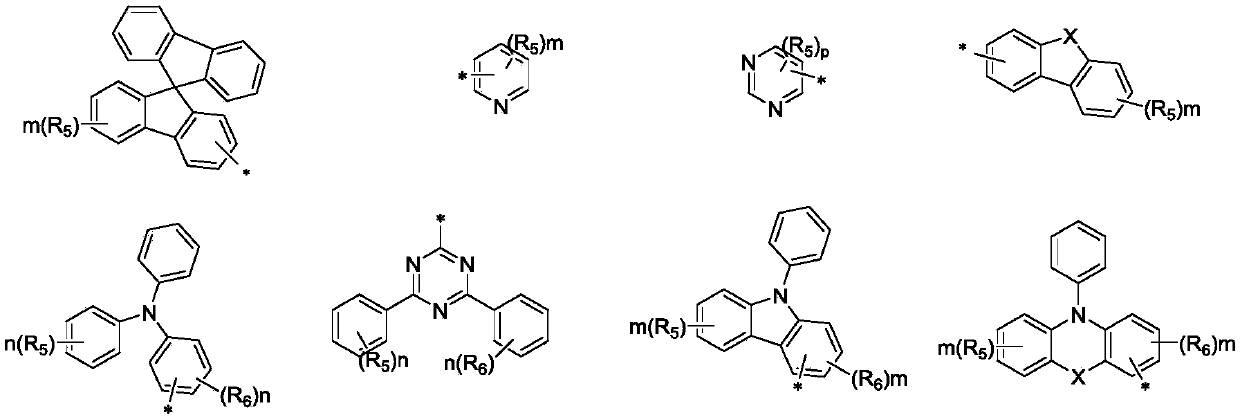
Spirofluorene nitrogen-containing heterocyclic ring organic electroluminescent material and organic light-emitting device thereof
A nitrogen-heterocyclic and luminescent technology, applied in luminescent materials, electrical solid devices, electrical components, etc., can solve the problems of exciton quenching and achieve the effects of increased stability, good luminous efficiency, and good blue light performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: the preparation of compound 93
[0068]
[0069] Step1. Take 100mmol of 93-4, add 1 equivalent of 0-2, 300mmol of sodium carbonate, 1mmol of tetrakistriphenylphosphopalladium, toluene, ethanol, water mixed solvent, replace with argon three times, react at reflux temperature for 5h, and the reaction ends Finally, deionized water was added, the organic phase was separated, washed three times with water, concentrated, and the obtained crude product was passed through a silica gel column to obtain 80 mmol of the product 93-3.
[0070] Step2. Add product 93-3 80mmol in the reaction vessel; iodobenzene 80mmol, potassium tert-butoxide 240mmol, Pd 2 (dba) 3 0.8mol, ultrasonic deoxygenated xylene, stirred to dissolve, replaced the air three times, added 4% tri-tert-butylphosphine ligand, 2.4mmol, replaced the air three times again, and refluxed for 6h. Cool to room temperature, add enough dichloromethane to completely dissolve the product, pass through a smal...
Embodiment 2
[0073] Embodiment 2: the preparation of compound 95
[0074] With embodiment 1, the iodobenzene in Step2 is replaced by
Embodiment 3
[0075] Embodiment 3: the preparation of compound 97
[0076] With embodiment 1, the iodobenzene in Step2 is replaced by
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com