Organic electroluminescent material and its preparation method and organic electroluminescent device
An electroluminescent material and electroluminescent technology, applied in the fields of luminescent materials, electro-solid devices, organic chemistry, etc., can solve problems such as the influence of the overall performance of the device, achieve a good maximum current efficiency, a wide range of raw material sources, and suitable for large-scale production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
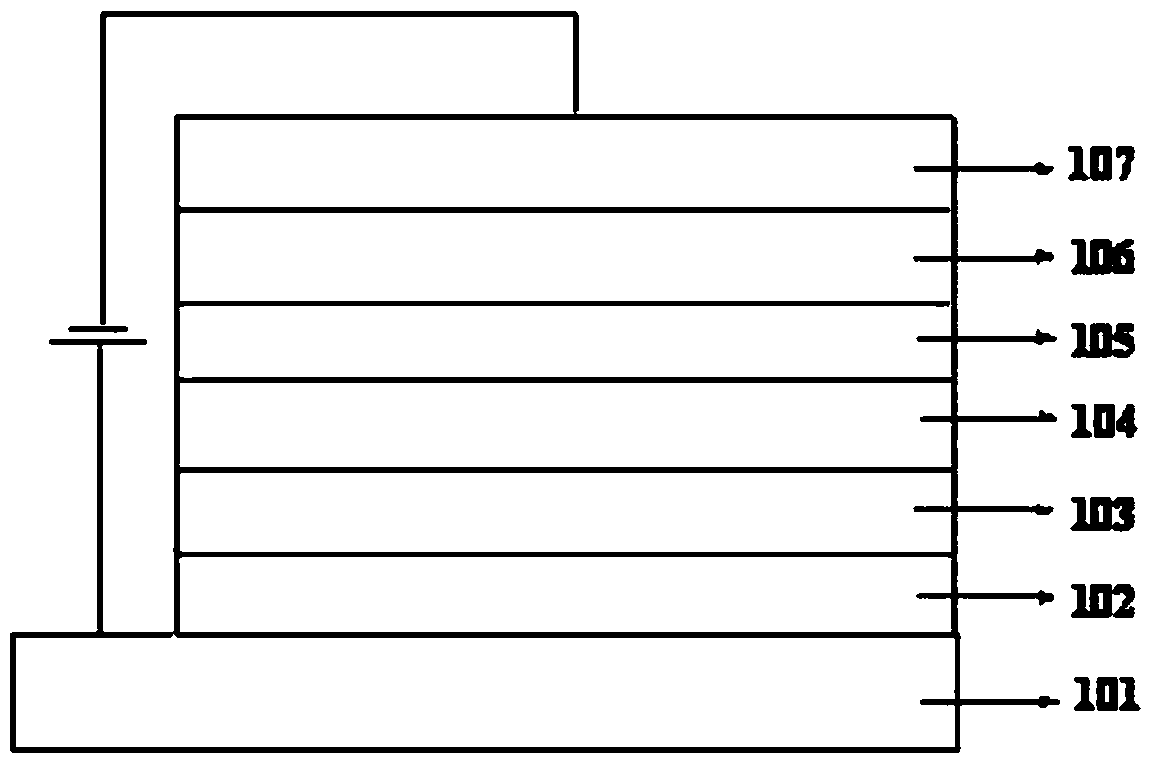
Image
Examples
Embodiment 1
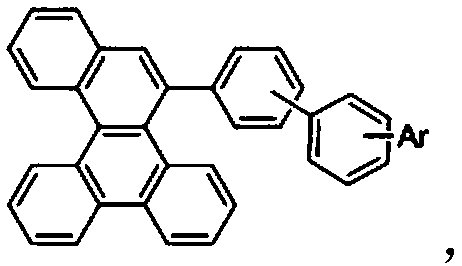
[0095] An organic electroluminescent material compound C01, which centers on the benzo[g]chrysene structure, introduces the first benzene ring into the g position of the benzo[g]chrysene structure, and then replaces the first benzene ring with a second benzene ring The hydrogen group on the benzene ring, and then replace the hydrogen group on the second benzene ring with a nitrogen-containing heterocycle, which has the following structure:
[0096]
[0097] A preparation method of organic electroluminescence material C01 as above, it comprises the following steps:
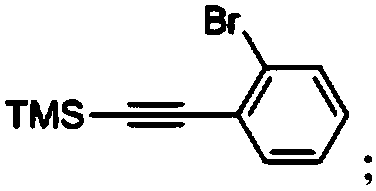
[0098] A, preparation of intermediate a
[0099] Under the protection of nitrogen, in a 500mL three-necked flask, dissolve o-bromoiodobenzene (42.4g, 0.15mol) and trimethylsilylacetylene (17.7g, 0.18mol) in 250mL triethylamine, and finally put tetrakistriphenylphosphine Combine palladium (5.1g, 4.5mmol) and cuprous iodide (1.71g, 9mmol), control the temperature at 20-25°C, stir for 48 hours, carry out a substit...
Embodiment 2
[0125] An organic electroluminescent material compound C05, its structure is similar to the compound C01 in Example 1, the difference is that the nitrogen-containing heterocyclic ring is different, and its structural formula is:
[0126]
[0127] A preparation method of organic electroluminescent material compound C05 as described above, its preparation method is similar to the preparation method of compound C01 in Example 1, the difference is:
[0128] In step F, prepare the organic electroluminescent material compound C05:
[0129] Under the protection of nitrogen, the intermediate e2 (1.08g, 2.5mmol) and 3-ThPyDA (1.14g, 2.625mmol) were dissolved in 20mL of toluene, then an aqueous solution of potassium carbonate (50mL, 0.2M), and finally put into 18-C -6 (0.1g, 0.3mmol) and tetrakistriphenylphosphine palladium (0.116g, 0.1mmol), control the temperature of the system to 80-85°C, stir for 48 hours, and perform a substitution reaction under the catalysis of the catalyst, t...
Embodiment 3
[0135] An organic electroluminescent material compound C08, its structure is similar to the compound C01 in Example 1, the difference is that the nitrogen-containing heterocycle is different, and its structural formula is:
[0136]
[0137] A preparation method of organic electroluminescent material compound C08 as described above, its preparation method is similar to the preparation method of compound C01 in Example 1, the difference is:
[0138] In step F, prepare the organic electroluminescent material compound C08:
[0139] Under nitrogen protection, intermediate e2 (1.08g, 2.5mmol) and 4-ThPyDA (1.14g, 2.625mmol) were dissolved in 30mL of toluene, then an aqueous solution of potassium carbonate (50mL, 0.2M) was added, and finally 18- C-6 (0.1g, 0.3mmol) and tetrakistriphenylphosphine palladium (0.116g, 0.1mmol), the temperature of the control system is raised to 80-85°C, stirred for 48 hours, and the substitution reaction is carried out under the catalysis of the catal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com