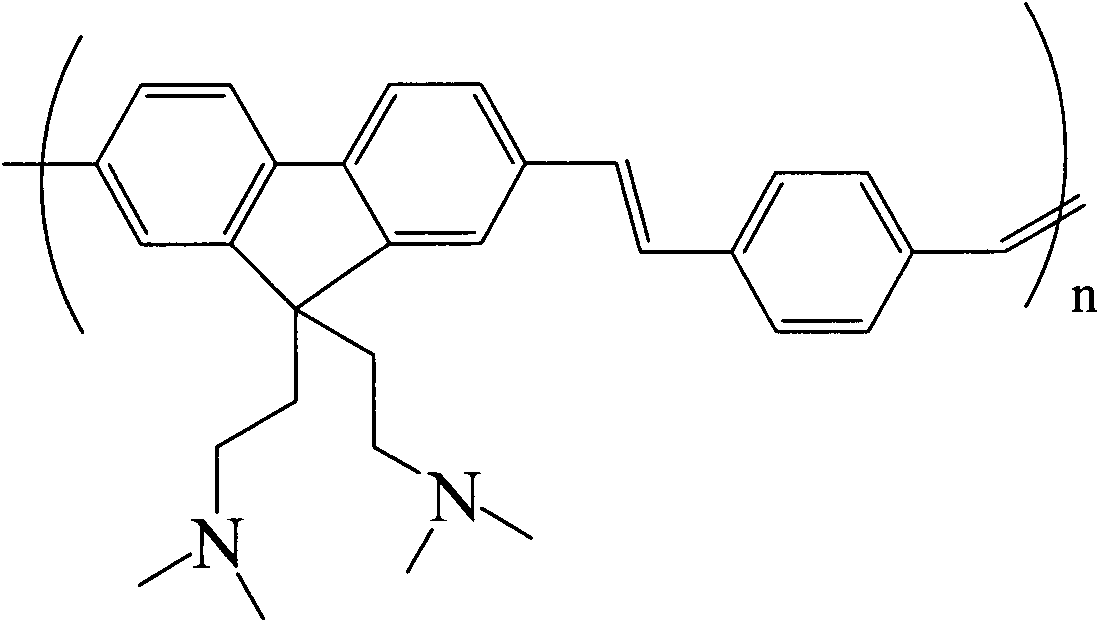
Polyfluorene/poly p-divinyl benzene and synthetic method thereof
A technology of divinylbenzene and polyfluorene, which is used in the design and synthesis of polyfluorene/poly-p-divinylbenzene, can solve the problems of large structure, poor flexibility, and inability to achieve conjugation of small molecular polymers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
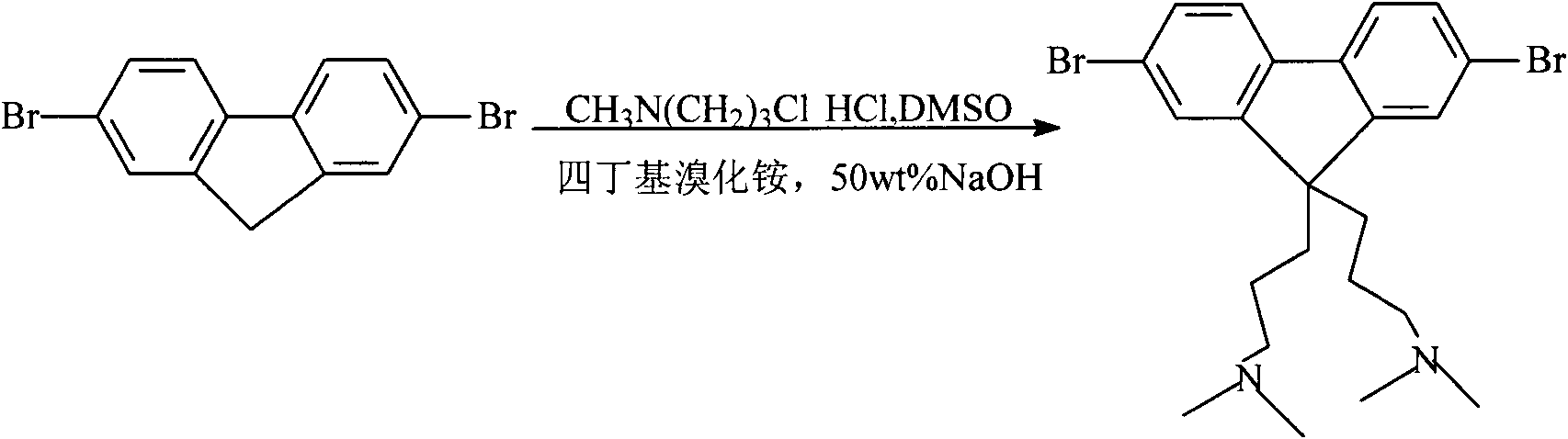
[0030] The first step: in a 250mL three-necked flask, under a nitrogen atmosphere, 4g 2,7-dibromofluorene (12mmol) and 80mg tetrabutylammonium bromide were added to 60mL DMSO, and after the solid was dissolved, 8ml of 50% mass fraction of NaOH solution. Dissolve 5g of 3-dimethylaminopropyl chloride hydrochloride ((CH 3 ) 2 N(CH 2 ) 3 (20 mL of Cl·HCl) (32 mmol) was added dropwise to the mixture, and the mixture was vigorously stirred at room temperature for 6 hours to stop the reaction. Water was added to the reaction to dissolve any salts. Extract, combine the organic layers, and wash with a certain mass fraction of NaOH solution, water and concentrated brine. The washed solution was dried with anhydrous MgSO4, filtered under reduced pressure, and the solvent was evaporated by rotary evaporation to obtain a yellow solid crude product, which was recrystallized from methanol / water to obtain white crystals (2.93 g, 48.2%).
[0031] IR (cm-1): 653 (C-Br), 1104 (C-N), 1607 (...
Embodiment 2
[0045] The first step: in a 250mL three-necked flask, under a nitrogen atmosphere, 4g 2,7-dibromofluorene (12mmol) and 80mg tetrabutylammonium bromide were added to 60mL DMSO, and after the solid was dissolved, 8ml of 50% mass fraction of NaOH solution. Dissolve 5g of 3-dimethylaminopropyl chloride hydrochloride ((CH 3 ) 2 N(CH 2 ) 3 (20 mL of Cl·HCl) (32 mmol) was added dropwise to the mixture, and the mixture was vigorously stirred at room temperature for 6 hours to stop the reaction. Water was added to the reaction to dissolve any salts. Extract, combine the organic layers, and wash with a certain mass fraction of NaOH solution, water and concentrated brine. The washed solution was washed with anhydrous MgSO 4 Drying, filtration under reduced pressure, and rotary evaporation to remove the solvent gave a crude product as a yellow solid, which was recrystallized from methanol / water to give white crystals (2.93 g, 48.2%).
[0046] IR (cm-1): 653 (C-Br), 1104 (C-N), 1607...
Embodiment 3
[0060] The first step: in a 250mL three-necked flask, under a nitrogen atmosphere, 4g 2,7-dibromofluorene (12mmol) and 80mg tetrabutylammonium bromide were added to 60mL DMSO, and after the solid was dissolved, 8ml of 50% mass fraction of NaOH solution. Dissolve 5g of 3-dimethylaminopropyl chloride hydrochloride ((CH 3 ) 2 N(CH 2 ) 3 (20 mL of Cl·HCl) (32 mmol) was added dropwise to the mixture, and the mixture was vigorously stirred at room temperature for 6 hours to stop the reaction. Water was added to the reaction to dissolve any salts. Extract, combine the organic layers, and wash with a certain mass fraction of NaOH solution, water and concentrated brine. The washed solution was washed with anhydrous MgSO 4 Dry, filter under reduced pressure, and remove the solvent by rotary evaporation to obtain a crude product of yellow homogeneity, which is recrystallized from methanol / water to obtain white crystals (2.93 g, 48.2%).
[0061] IR (cm-1): 653 (C-Br), 1104 (C-N), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com