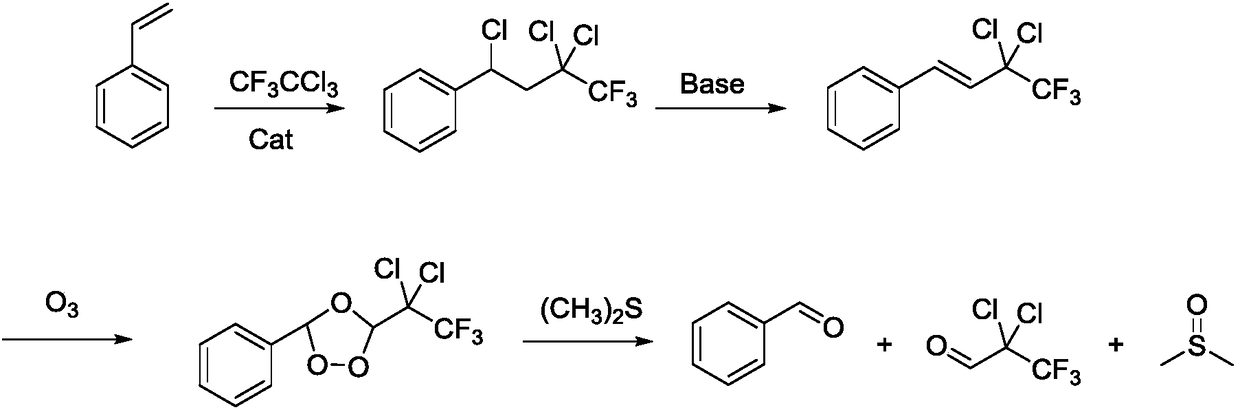
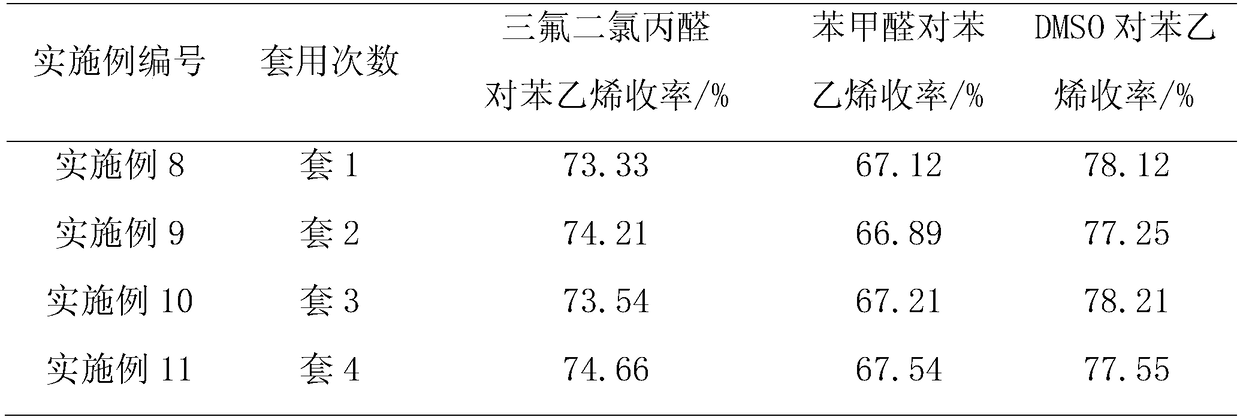
Method for synthesizing pesticide intermediate 3,3,3-trifluoro-2,2-dichloropropylaldehyde
A technology of dichloropropionaldehyde and trifluorotrichloroethane, which is applied in chemical instruments and methods, organic chemistry, preparation of halogenated hydrocarbons, etc., and can solve problems such as low yield of target products, difficulty in industrialization, low utilization rate of raw materials, etc. problem, to achieve the effect of high product yield, easy industrialization, and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
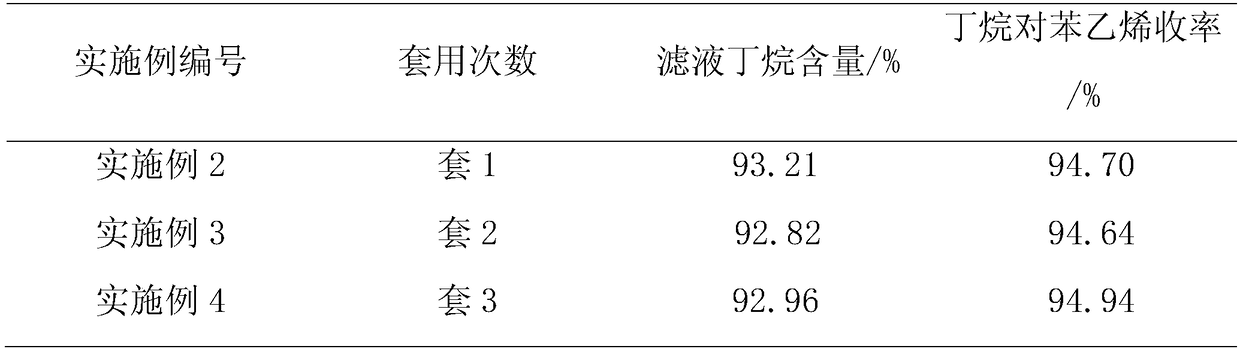
[0034] Put 113.00 g of N,N-dimethylformamide, 113.00 g of styrene, 407.01 g of trifluorotrichloroethane, and 4.07 g of copper chloride powder into a 1L four-neck bottle, and heat up to 120°C for 6 hours of reaction. After the reaction was completed, gas spectrum quantitative analysis of the still material showed that styrene was 0.02%, butane was 47.11%, the conversion rate of styrene was 99.89%, and the yield of butane to styrene was 94.30%. The reaction solution was distilled under normal pressure and negative pressure, and the distillation fraction was 334.13g, which was applied mechanically; the temperature was lowered, and 8.20g of catalyst was recovered by filtration, which was applied mechanically; the filtrate was 320.12g, the butane content was 93.35%, and the yield of styrene was 93.20%.
Embodiment 2-4
[0036]The distillation fraction (containing trifluorotrichloroethane and N,N-dimethylformamide) and the recovered catalyst of Example 1 were applied mechanically. Taking set 1 as an example, put in 334.13g of the distilled fraction in Example 1, add 214.00g of trifluorotrichloroethane, 113.00g of styrene, then put in 8.21g of recovered catalyst and 0.20g of new catalyst, and heat up to 120°C for reaction 6 hours. After the heat preservation was completed, the temperature was lowered, and the aftertreatment was the same as that in Example 1 to obtain 8.62 g of recovered catalyst, 321.23 g of filtrate, 93.20% of butane content in the filtrate, and 94.70% of styrene yield for feeding.
Embodiment 3
[0037] Example 3 (set 2) The distilled fraction and recovered catalyst of example 2 (set 1) were used mechanically, new catalyst was supplemented, trifluorotrichloroethane and styrene were added, and the reaction was carried out at 120° C. for 6 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com