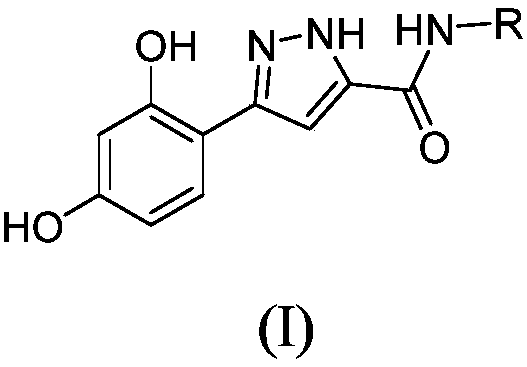
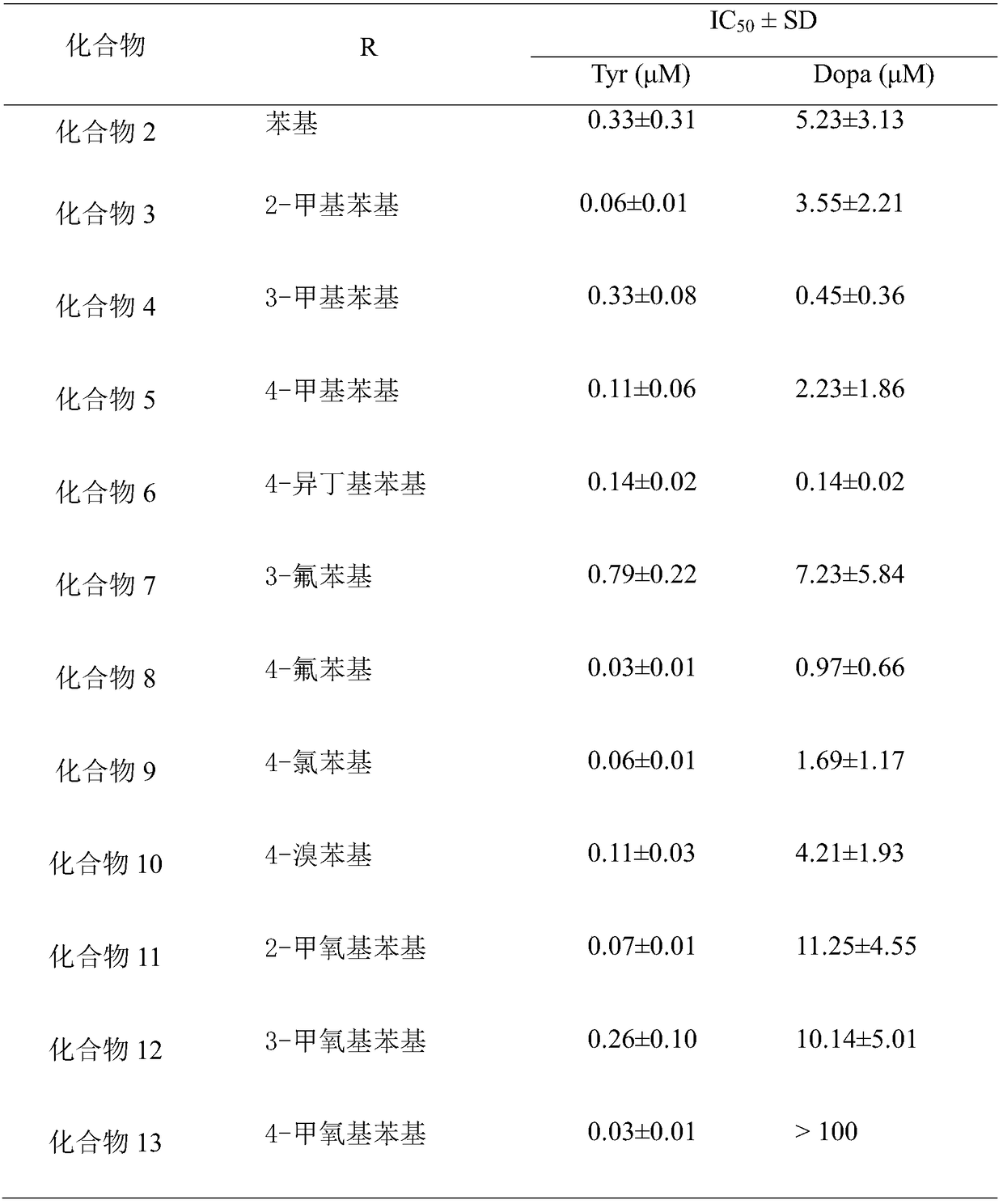
Tyrosinase inhibitor, and preparation method and applications thereof
A compound and pharmaceutical technology, applied in the field of medicine, can solve problems such as restricted use and safety issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
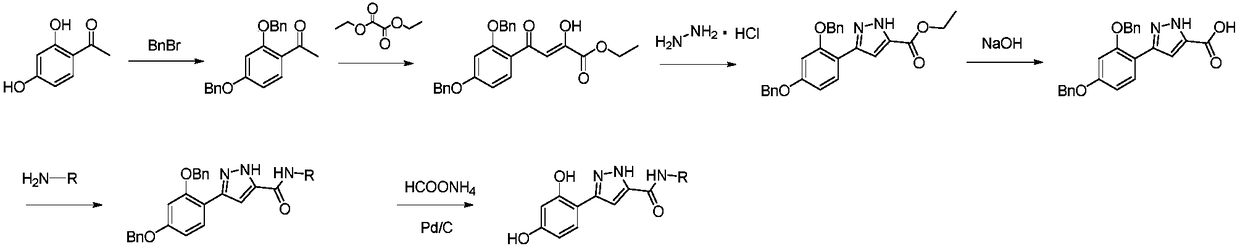
[0053] (1) Synthesis of 1-(2,4-bis(benzyloxy)phenyl)ethanone
[0054] Take 2,4-dihydroxyacetophenone (5g, 32.8mmol) and benzyl bromide (14.1g, 82.2mmol), add it to an eggplant-shaped flask, add acetonitrile, heat in an oil bath at 85°C, and reflux the reaction solution for 3-5 Hours, after the reaction is over, spin off the acetonitrile and add water, extract with ethyl acetate, wash with brine, dry the organic layer, recrystallize, filter with suction, and dry the filter cake under infrared to obtain a white solid with a yield of 95%.
[0055] (2) Synthesis of ethyl 4-(2,4-bis(benzyloxy)phenyl)-2,4-dioxobutanoate
[0056] Take 1-(2,4-bis(benzyloxy)phenyl)ethanone (8g, 24.1mmol), diethyl oxalate (4.22g, 28.9mmol) and sodium ethoxide (3.28g, 48.1mmol), add to Add ethanol to an eggplant-shaped bottle, heat in an oil bath at 80°C, reflux the reaction solution for 5-8 hours, and cool to room temperature; a yellow solid precipitates, is suction filtered, and the filter cake is dri...
Embodiment 2
[0069] Preparation of 3-(2,4-dihydroxyphenyl)-N-(o-tolyl)-1H-pyrazole-5-carboxamide:
[0070] Referring to the synthesis method of Example 1, the intermediate 1 of Example 1 was replaced by 3-(2,4-bis(benzyloxy)phenyl)-N-(o-tolyl)-1H-pyrazole-5-methyl Amide, to obtain a white solid compound, which was detected as one point by TLC, with fluorescence at 254nm and no fluorescence at 365nm under a UV lamp.
[0071] 1 H NMR(300MHz,DMSO-d6)δ13.16(s,1H),10.18(s,1H),9.61(s,1H),9.39(s,1H),7.71(s,1H),7.48(d, J=8.3Hz, 1H), 7.24(m, 2H), 7.11(s, 1H), 7.01(s, 1H), 6.45(s, 1H), 6.34(d, J=8.3Hz, 1H), 2.28( s,3H).ESI-MS m / z 310.1211[M+H] + .
Embodiment 3
[0073] Preparation of 3-(2,4-dihydroxyphenyl)-N-(m-tolyl)-1H-pyrazole-5-carboxamide:
[0074] Referring to the synthesis method of Example 1, the intermediate 1 of Example 1 was replaced by 3-(2,4-bis(benzyloxy)phenyl)-N-(m-tolyl)-1H-pyrazole-5-methyl Amide, to obtain a white solid compound, which was detected as one point by TLC, with fluorescence at 254nm and no fluorescence at 365nm under a UV lamp.
[0075] 1 H NMR (300MHz, DMSO-d6) δ9.92(s, 2H), 7.69–7.55(m, 2H), 7.49(d, J=8.5Hz, 1H), 7.23(t, J=7.7Hz, 1H) ,7.14(s,1H),6.91(d,J=7.2Hz,1H),6.45(d,J=2.2Hz,1H),6.35(dd,J=8.5,2.3Hz,1H),2.31(s, 3H).ESI-MS m / z 310.1203[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com