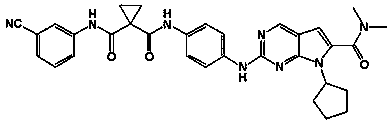
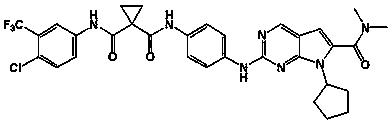
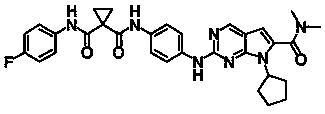
Preparation and applications for novel 1,1-cyclopropyl diamide derivative
A technology of cyclopropyl diamide and derivatives, which is applied in the direction of medical preparations containing active ingredients, drug combinations, organic active ingredients, etc., can solve the problem of preparation and application of 1,1-cyclopropyl diamide derivatives, which has not been reported yet And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The starting materials used in the preparation of the compounds of the present invention are known, can be prepared according to known methods, or are commercially available.
[0019] The invention also relates to novel intermediates and / or starting materials. Particular preference is given to reaction conditions and novel intermediates which are the same or similar to those mentioned in the examples.
[0020] Both intermediates and final products can be worked up and / or purified according to conventional methods including pH adjustment, extraction, filtration, drying, concentration, chromatography, trituration, crystallization, and the like.
[0021] In addition, the compounds of the present invention can be prepared by various methods known in the art or variations on the methods described herein.
[0022] The following examples are only used to illustrate the present invention and do not limit the present invention in any way.
Embodiment 1
[0023] Example 1 N-(4-(7-cyclopentyl-6-(dimethylcarbamoyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl)amino)phenyl)-N -Synthesis of phenylcyclopropane-1,1-dicarboxamide
[0024]
[0025] Step 1.1: Preparation of 1-(phenylcarbamoyl)cyclopropane-1-carboxylic acid
[0026]
[0027] Cyclopropane-1,1-dicarboxylic acid (13.01 g, 100 mmol) was dissolved in 150 mL of isopropyl acetate and cooled to 0°C, then thionyl chloride (12.5 g, 105 mmol) was added dropwise. After the dropwise addition was completed, the mixture was raised to room temperature and stirred for 6 hours. A solution of aniline (110 mmol) and triethylamine (15.29 mL, 110 mmol) in isopropyl acetate (40 mL) was added dropwise for about 1 hour, and stirring was continued for 2 hours. The reaction solution was diluted with ethyl acetate (500 mL), washed with 1 N hydrochloric acid and saturated sodium chloride, dried over anhydrous magnesium sulfate and concentrated to obtain a crude product. Suspend the crude product in hept...
Embodiment 2
[0034] Example 2 N-(4-(7-cyclopentyl-6-(dimethylcarbamoyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl)amino)phenyl)-N Synthesis of -(2-fluorophenyl)cyclopropane-1,1-dicarboxamide
[0035]
[0036] Step 2.1: 1 - Synthesis of ((2-fluorophenyl)carbamoyl)cyclopropane-1-carboxylic acid
[0037]
[0038] The method is the same as step 1.1, white solid, yield: 45%, 1 H NMR (400 MHz, DMSO-d 6 ) δ 13.51 (s,1H), 11.27 (s, 1H), 8.15 (td, J = 8.0, 2.0 Hz, 1H), 7.27 (ddd, J = 11.4, 7.9,1.8 Hz, 1H), 7.13 (tdd, J = 13.3, 7.7, 3.7 Hz, 2H), 1.56 (h, J = 4.0 Hz, 4H).
[0039] Step 2.2: Synthesis of N-(4-aminophenyl)-N-(2-fluorophenyl)cyclopropane-1,1-dicarboxamide
[0040]
[0041] The method is the same as step 1.2, brown solid, yield: 54%. 1 H NMR (400 MHz, DMSO-d 6 ) δ 11.16 (s,1H), 9.40 (s, 1H), 8.04 (td, J = 7.8, 2.4 Hz, 1H), 7.33 – 7.22 (m, 1H), 7.20– 7.09 (m, 4H), 6.53 ( d, J = 8.4 Hz, 2H), 4.99 (s, 2H), 1.66 – 1.53 (m, 4H); 13 C NMR (101 MHz, DMSO) Δ 170.14, 169.01, 152.43, 146.32...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com