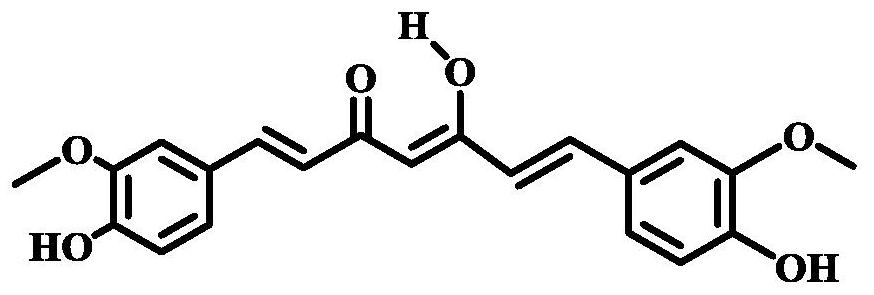
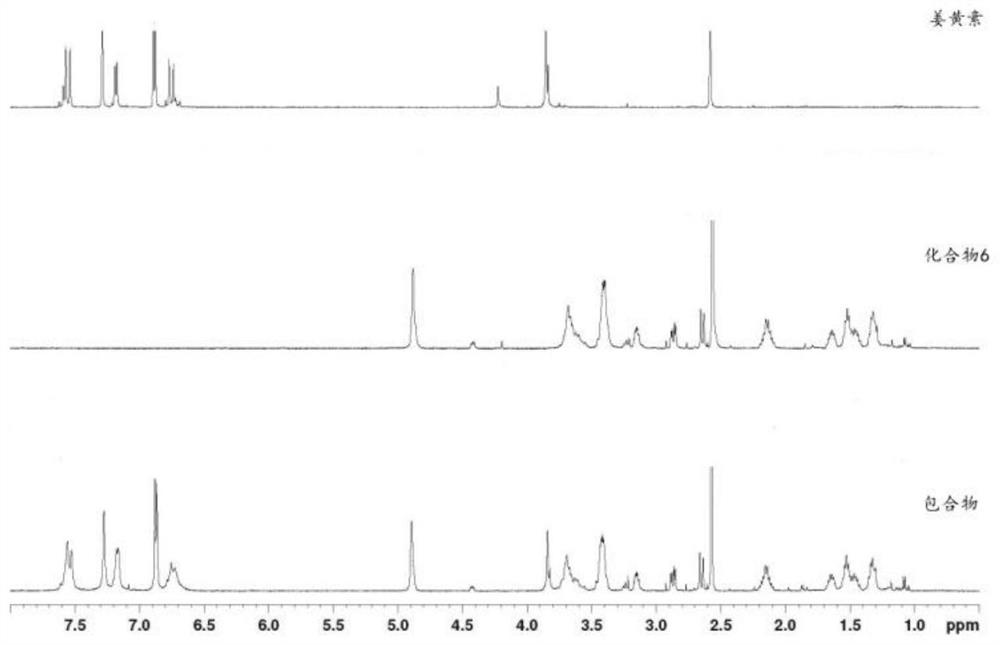
Mono-6-(biotinamide)-6-deoxy-β-cyclodextrin and its preparation method and application
A technology based on biotinamide and cyclodextrin, which is applied in the direction of drug combination, ketone active ingredients, antineoplastic drugs, etc., can solve the problems affecting the delivery of curcumin, improve the antitumor efficiency, expand the hydrophobic cavity, reduce the side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
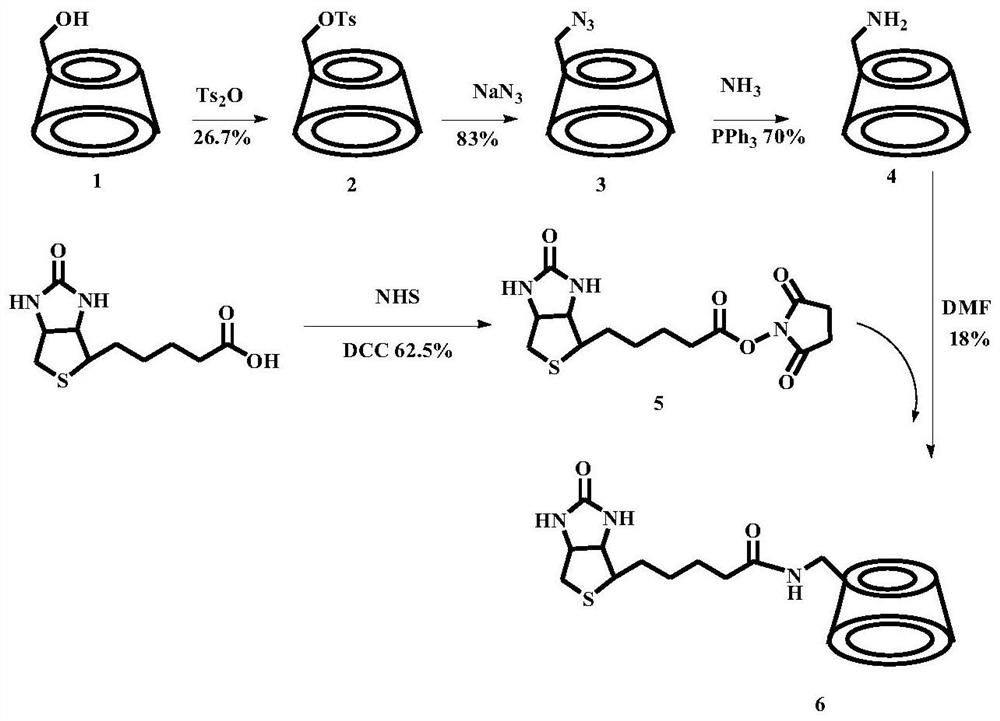
[0041] Example 1 Preparation of single (6-oxo-6-p-toluenesulfonyl)-β-cyclodextrin (2)
[0042] β-cyclodextrin (1) (9.99g, 88mmol) was dissolved in 220ml of water, added finely ground Ts 2 O (4.26g, 13mmol) crystallized and stirred in an ice bath for 2h. 10% NaOH aqueous solution was added dropwise to the reaction solution, stirred for 20 min and then filtered. use NH 4 Cl adjusted the pH value of the filtrate to neutral, and a large amount of white solids were precipitated. They were refrigerated overnight in the refrigerator, and 2.97 g of white solids were obtained by filtration, with a yield of 26.1%. ESI / MS(m / z):1290.30[M+H] + .
Embodiment 2
[0043] Example 2 Preparation of mono-(6-azido-6-deoxy)-β-cyclodextrin (3)
[0044] 6-Oxygen-6-p-toluenesulfonyl)-β-cyclodextrin (2) (1.34g, 1mmol) and sodium azide
[0045] (0.82g, 12.3mmol) was dissolved in 13.5ml of distilled water, and the reaction was stirred in an oil bath at 80°C (reflux by condensation). After 5 hours, it was taken out from the eggplant-shaped bottle and cooled to room temperature, and filtered to obtain a white solid powder (1.02 g, 83%). ESI-MS(m / s): 1187.40[M+H] + .
Embodiment 3
[0046] Example 3 Preparation of mono-(6-amino-6-deoxy)-β-cyclodextrin (4)
[0047] Mono-(6-azido-6-deoxy)-β-cyclodextrin (3) (1.21g, 1mmol) was dissolved in 5ml DMF and added to a 50ml eggplant-shaped bottle, and three Phenylphosphonium (0.58g, 2.2mmol). Then 3.5ml of concentrated ammonia water was added dropwise, and a white solid powder (0.81g, 70%) was obtained by filtration. ESI-MS(m / s): 1135.20[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com