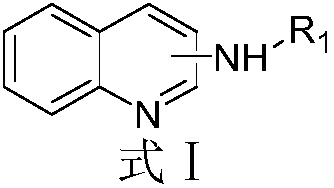
Aminoquinoline compounds, and preparation method and application thereof
An aminoquinoline and compound technology, applied in the field of medicine, can solve the problems of hepatotoxicity, azole drug resistance, clinical application limitation and the like, and achieve the effect of good antifungal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
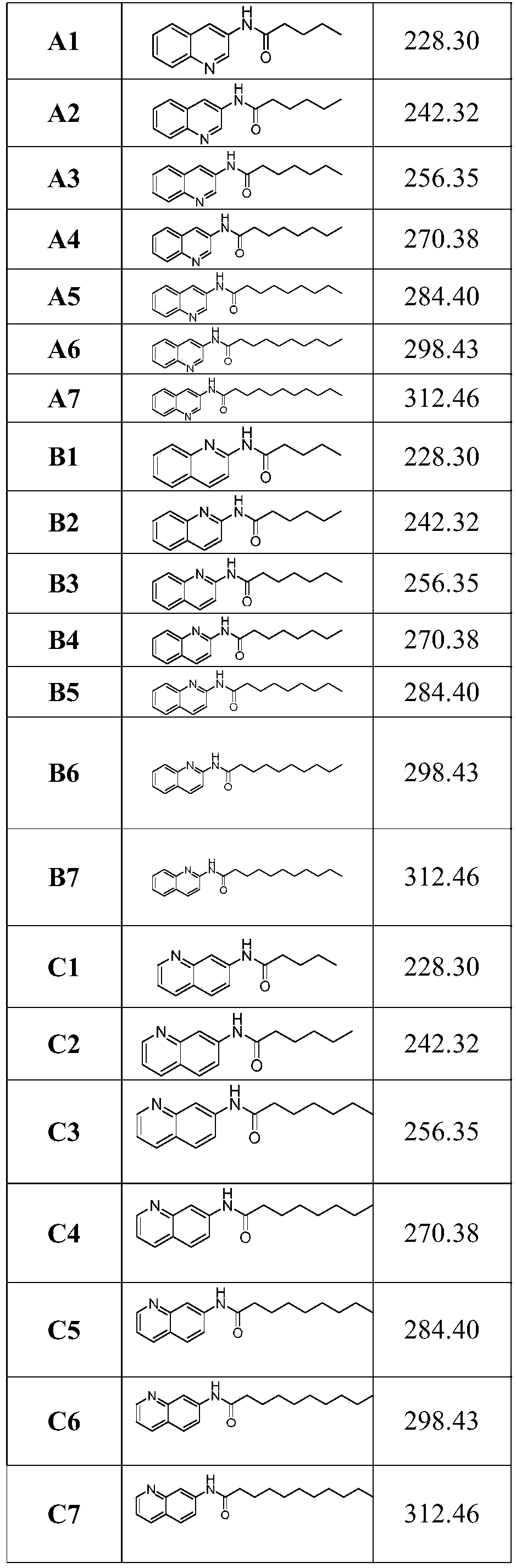
[0033] Embodiment 1. Preparation of compound A1
[0034] Weigh 3-aminoquinoline (144mg, 1mmol), add 15ml of acetonitrile to dissolve, add N,N-diisopropylethylamine DIPEA (157mg, 1.2mmol) and valeryl chloride (145mg, 1.2mmol) successively, mix well Stir overnight at room temperature.
[0035] After the reaction was completed, the solvent acetonitrile was removed under reduced pressure, an appropriate amount of water (10ml) was added, and the aqueous layer (20ml*3) was extracted with ethyl acetate, and the ethyl acetate layer was washed twice with saline; Ether: ethyl acetate = 1:1 mixed solvent was used as the eluent for column chromatography to obtain 116 mg of the product.
[0036] The hydrogen spectrum of A1 is as follows:
[0037] 1 H NMR (400MHz, CDCl 3 )δ8.83(s,1H),8.77(d,J=2.4Hz,1H),8.02(t,J=12.7Hz,1H),7.92-7.76(m,2H),7.63(t,J=7.6 Hz,1H),7.54(t,J=7.5Hz,1H),2.43(dt,J=30.5,7.5Hz,2H),1.86–1.66(m,2H),1.57-1.34(m,2H),1.01 -0.89(m,3H).
Embodiment 2
[0038] Embodiment 2. Preparation of compound A2
[0039] Weigh 3-aminoquinoline (144mg, 1mmol), add 15ml of acetonitrile to dissolve, add N,N-diisopropylethylamine DIPEA (144mg, 1.1mmol) and hexanoyl chloride (148mg, 1.1mmol) successively, mix well Stir overnight at room temperature.
[0040] After the reaction was completed, the solvent acetonitrile was removed under reduced pressure, an appropriate amount of water (10ml) was added, and the aqueous layer (20ml*3) was extracted with ethyl acetate, and the ethyl acetate layer was washed twice with saline; Ether: ethyl acetate = 1:1 mixed solvent was used as the eluent for column chromatography to obtain 124 mg of the product.
[0041] The hydrogen spectrum of A2 is as follows:
[0042] 1 H NMR (400MHz, CDCl 3 )δ8.79(t, J=9.6Hz, 2H), 8.02(d, J=7.6Hz, 2H), 7.78(d, J=8.2Hz, 1H), 7.57(dt, J=38.4, 7.5Hz, 1H), 2.96(s, 1H), 2.89(s, 1H), 2.52-2.08(m, 2H), 1.78(dd, J=14.1, 7.2Hz, 2H), 1.33(dd, J=23.0, 20.1Hz ,4H),0.90(t,J=6.0Hz,3H...
Embodiment 3
[0043] Embodiment 3. Preparation of compound A3
[0044] Weigh 3-aminoquinoline (144mg, 1mmol), add 15ml of acetonitrile to dissolve, add N,N-diisopropylethylamine DIPEA (144mg, 1.1mmol) and heptanoyl chloride (178mg, 1.2mmol) successively, mix well Stir overnight at room temperature.
[0045] After the reaction was completed, the solvent acetonitrile was removed under reduced pressure, an appropriate amount of water (10ml) was added, and the aqueous layer (20ml*3) was extracted with ethyl acetate, and the ethyl acetate layer was washed twice with saline; Ether: ethyl acetate = 1:1 mixed solvent was used as the eluent for column chromatography to obtain 130 mg of the product.
[0046] The hydrogen spectrum of A3 is as follows:
[0047] 1 H NMR (400MHz, CDCl 3)δ8.91-8.60 (m, 3H), 7.99 (d, J = 8.4Hz, 1H), 7.73 (d, J = 8.1Hz, 1H), 7.59 (dd, J = 8.2, 7.1Hz, 1H), 7.49(t, J=7.5Hz, 1H), 2.39(dt, J=24.0, 7.5Hz, 2H), 1.79-1.54(m, 2H), 1.44-1.08(m, 6H), 0.94-0.68(m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com