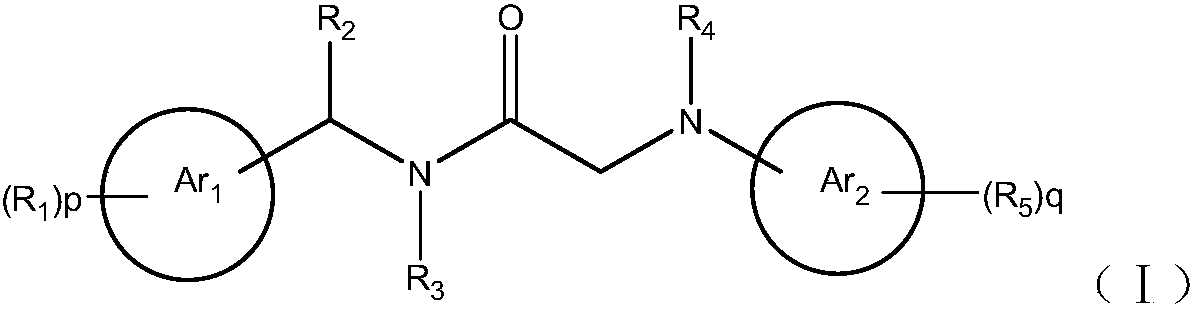
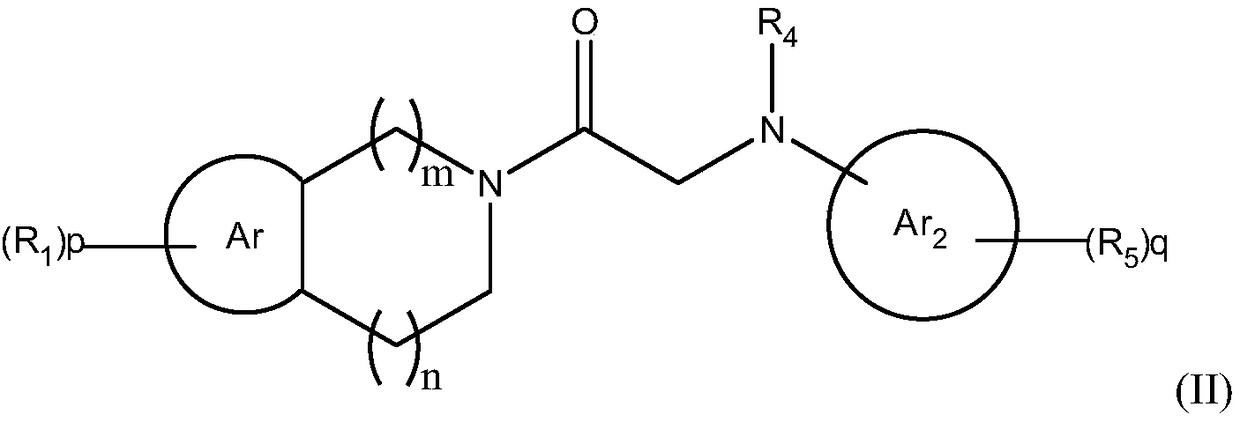
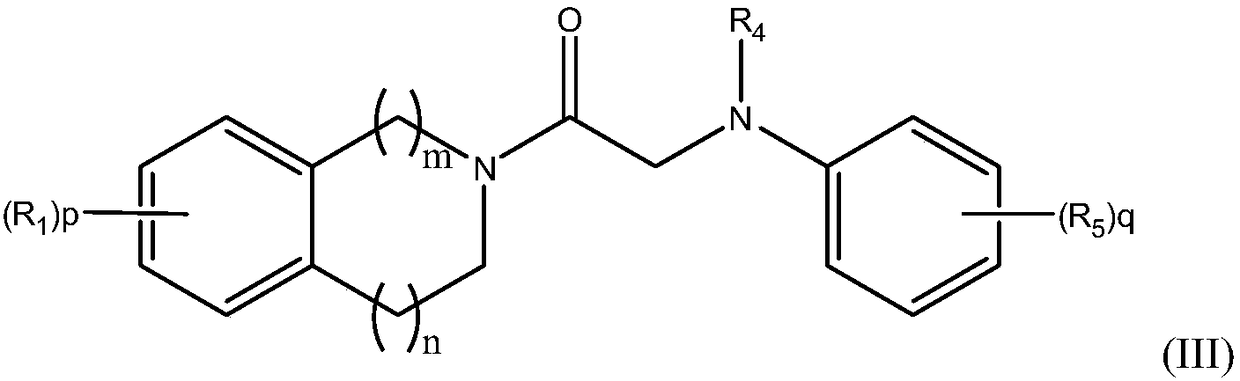
Amide compound for prevention and treatment of mental disorders
A compound and solvate technology, which is applied in the field of amide compounds for the prevention and treatment of mental disorders, can solve problems such as slow onset of action, onset of toxic and side effects, and effects on post-synaptic membrane function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Example 1 2-(2-((2-(3,4-dihydroisoquinolin-2(1H)-yl)-2-oxoethyl)amino)phenyl)acetic acid Preparation of methyl ester (compound 1)
[0129]
[0130] Step 1. 2-Bromo-1-(3,4-dihydroisoquinolin-2(1H)-yl)ethanone
[0131] Dissolve tetrahydroisoquinoline (5.0g, 37.54mmol), N,N-dimethylaniline (5.3mL, 41.29mmol) in dichloromethane (80mL), bromoacetyl bromide (3.6mL, 41.29mmol) in Add dropwise to the reaction solution at 0-5°C. After the dropwise addition was completed, the stirring was continued for 30 min, and the reaction was monitored by TLC to end. The reaction solution was poured into ice water, the organic phase was separated, and the aqueous phase was extracted with dichloromethane. The combined organic phases were washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated. The crude product was purified by silica gel column chromatography (petroleum ether / ethyl acetate=7 / 1) to obtain 2-bromo-1-(3,4-dihydroisoquinolin-2...
Embodiment 2-15
[0135] Example 2-15 Synthesized Example 2-15 with reference to the method of Example 1
[0136]
[0137]
[0138]
Embodiment 16
[0139] Example 16 2-(3-(N-(2-(3,4-dihydroisoquinolin-2(1H)-yl)-2-oxoethyl)methylsulfonamide base) phenyl) methyl acetate
[0140]
[0141] Step 1. Methyl 2-(3-(methylsulfonylamino)phenyl)acetate
[0142] 2-(3-Aminophenyl)methyl acetate (300mg, 1.82mmol), pyridine (160mg, 2.00mmol) were dissolved in dichloromethane (10mL), and methylsulfonyl chloride (230mg, 2.00 mmol) was slowly added dropwise into the reaction solution. After 1 hour, TLC monitored the completion of the reaction. The reaction solution was poured into ice water, the organic phase was separated, the aqueous phase was extracted with dichloromethane, the combined organic phase was washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 2-(3-(methylsulfonylamino )phenyl)methyl acetate (450 mg, crude) as a pale yellow oil.
[0143] Step 2. 2-(3-(N-(2-(3,4-dihydroisoquinolin-2(1H)-yl)-2-oxoethyl)methylsulfonylamino)benzene base) methyl acetate
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com