Stable carbocisteine pharmaceutical composition
A technology of carbocisteine and a composition, which is applied in the field of carbocisteine pharmaceutical compositions, can solve the problems of not involving the effect of stabilizing carbocisteine, low solubility of carbocisteine, shortening the disintegration time of solid preparations, and the like, Achieve excellent long-term stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, carbocisteine pharmaceutical composition
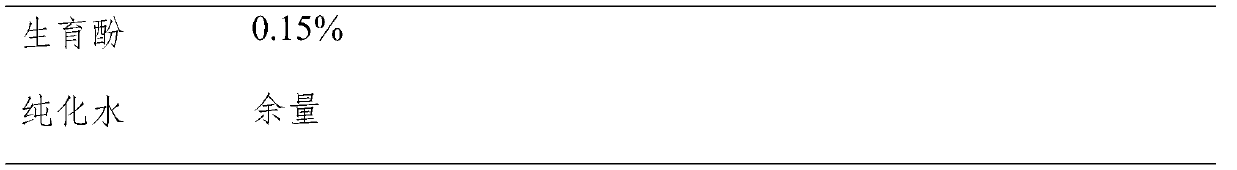
[0029]
[0030]
[0031] Preparation method: Dissolve sodium hydroxide in partially purified water to prepare a sodium hydroxide solution, take the prescribed amount of carbocisteine, dissolve it in partially purified water, slowly add the above sodium hydroxide solution dropwise, and stir until the carbocisteine Dissolve completely, add the tocopherol of prescription quantity, add the prescription quantity after fully stirring: hydroxypropyl cellulose, D-sodium gluconate and lactose, fully stir to make it dissolve completely, measure solution pH, add water to constant volume, filter, Sterilize, that is.
Embodiment 2
[0032] Embodiment 2, carbocisteine pharmaceutical composition
[0033]
[0034] The preparation method refers to Example 1.
Embodiment 3
[0035] Embodiment 3, carbocisteine pharmaceutical composition
[0036]
[0037] The preparation method refers to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com