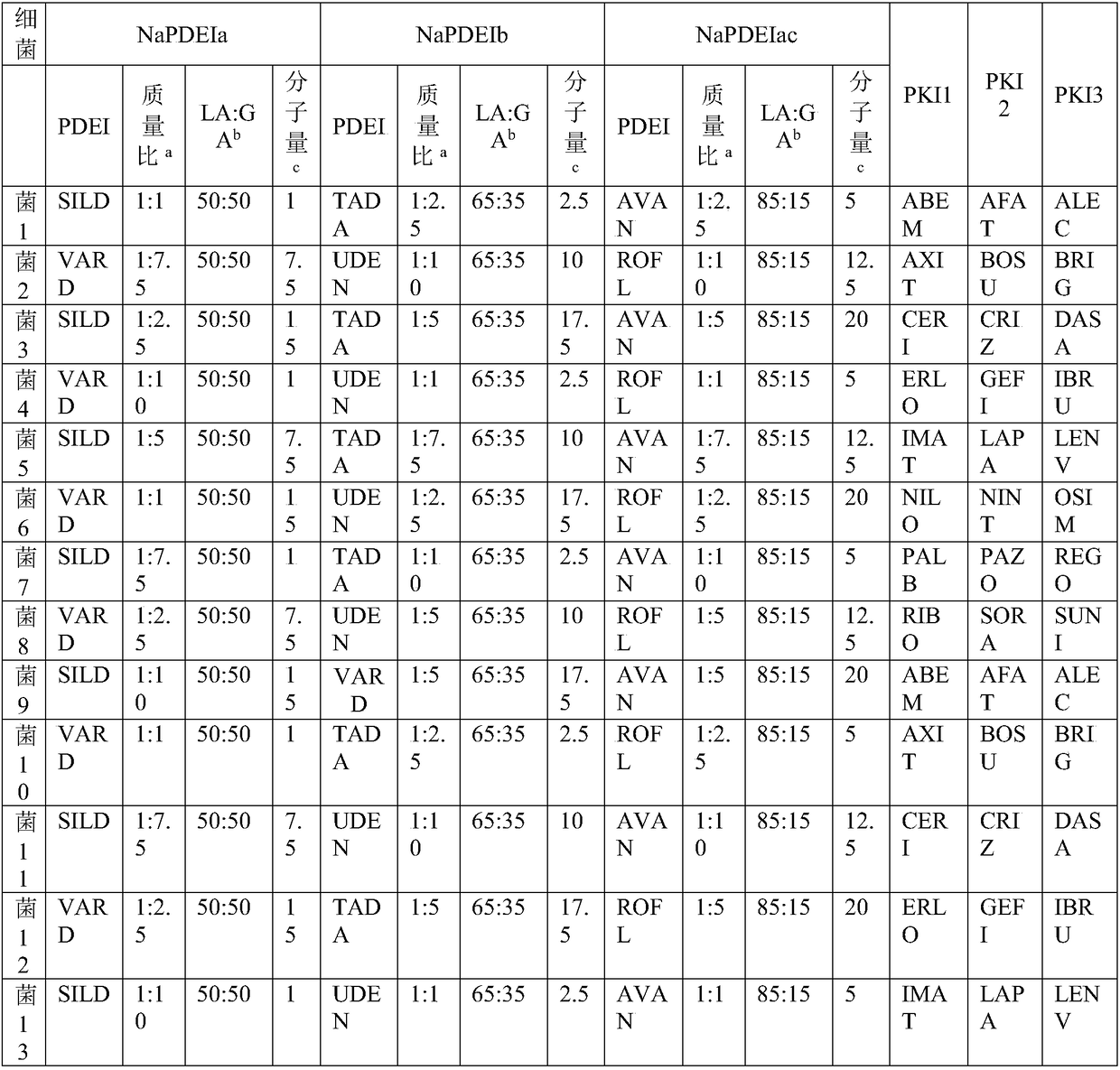
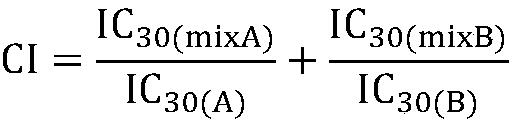Composition containing phosphodiesterase inhibitor nanoparticle and protein kinase inhibitor
A protein kinase inhibitor and phosphodiesterase technology, applied in the field of medicine, can solve the synergistic antibacterial effect of non-phosphodiesterase inhibitors, synergistic antibacterial effects of non-phosphodiesterase inhibitors and protein kinase inhibitors, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] 4. Preparation and structure characterization of nanoparticles
[0028] In the present invention, the phosphodiesterase inhibitor nanoparticles are prepared by a solvent evaporation method well known to those skilled in the art, and the preparation method is as follows:
[0029] Accurately weigh a certain mass ratio of the mixture of phosphodiesterase inhibitors and PLGA (total mass is 60g) and dissolve it in 200mL of ethanol, and ultrasonically sonicate for 2 minutes to fully dissolve to form an oil phase; Inject into 400mL polyvinyl alcohol aqueous solution with a concentration of 5mg / mL, stir at 2000rpm for 2min to form the first emulsion; then inject the first emulsion into 1000mL polyvinyl alcohol aqueous solution with a concentration of 5mg / mL, stir at 500rpm After 5 hours until the organic solvent evaporated completely, the second emulsion was formed; finally, it was centrifuged at 4000 rpm for 20 minutes, and washed twice with deionized water. The particles wer...
Embodiment 1
[0068] Example 1 Preparation of particles containing phosphodiesterase inhibitor nanoparticles and protein kinase inhibitors
[0069] prescription
[0070]
[0071]
[0072] Preparation
[0073] Accurately weigh a certain mass ratio of the mixture of phosphodiesterase inhibitors and PLGA (total mass is 60g) and dissolve it in 200mL of ethanol, and ultrasonically sonicate for 2 minutes to fully dissolve to form an oil phase; Inject into 400mL polyvinyl alcohol aqueous solution with a concentration of 5mg / mL, stir at 2000rpm for 2min to form the first emulsion; then inject the first emulsion into 1000mL polyvinyl alcohol aqueous solution with a concentration of 5mg / mL, stir at 500rpm After 5 hours until the organic solvent evaporated completely, the second emulsion was formed; finally, it was centrifuged at 4000 rpm for 20 minutes, and washed twice with deionized water. The particles were collected and vacuum-dried at room temperature for 12 hours to obtain white nanopa...
Embodiment 2
[0075] Example 2 Preparation of Capsules Containing Phosphodiesterase Inhibitor Nanoparticles and Protein Kinase Inhibitors
[0076] prescription
[0077]
[0078] Accurately weigh a certain mass ratio of the mixture of phosphodiesterase inhibitors and PLGA (total mass is 60g) and dissolve it in 200mL of ethanol, and ultrasonically sonicate for 2 minutes to fully dissolve to form an oil phase; Inject into 400mL polyvinyl alcohol aqueous solution with a concentration of 5mg / mL, stir at 2000rpm for 2min to form the first emulsion; then inject the first emulsion into 1000mL polyvinyl alcohol aqueous solution with a concentration of 5mg / mL, stir at 500rpm After 5 hours until the organic solvent evaporated completely, the second emulsion was formed; finally, it was centrifuged at 4000 rpm for 20 minutes, and washed twice with deionized water. The particles were collected and vacuum-dried at room temperature for 12 hours to obtain white nanoparticles, which were sealed and prot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com