Method and composition for treating cancer, killing metastatic cancer cells and preventing cancer metastasis using antibody to advanced glycation end products (AGE)
A technology for cancer metastasis and cancer cells, applied in chemical instruments and methods, drug combinations, antibody medical components, etc., and can solve problems such as inability to inhibit S phase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
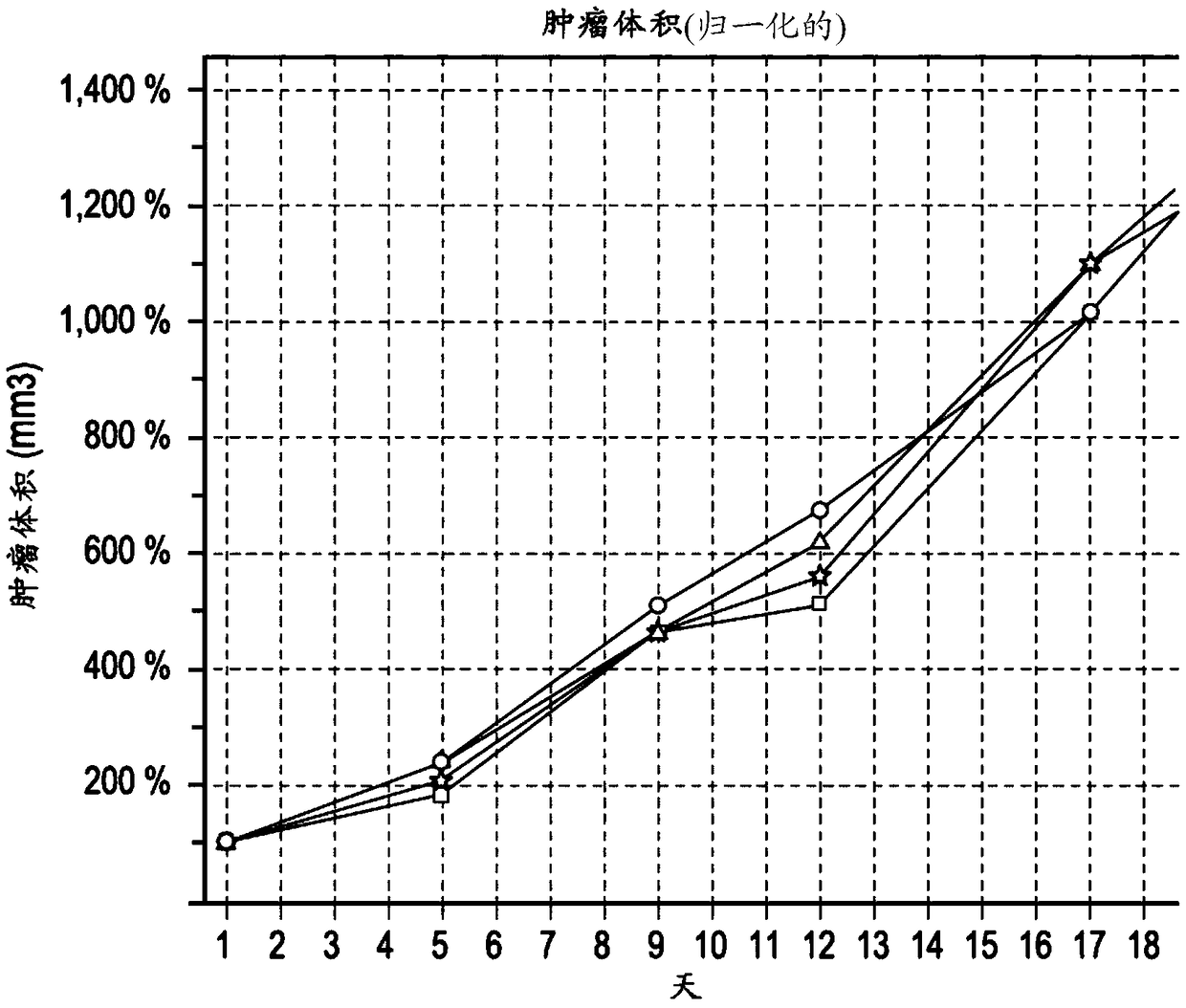
[0141] Example 1: In vivo study of administration of anti-glycation end product antibodies. This example demonstrates that anti-AGE antibodies can target cells that have AGE-modified proteins on the cell surface. Although the cells considered in this study are senescent cells, they can be considered as a model of metastatic cancer cells.
[0142] To examine the effect of anti-glycation end product antibodies, the antibodies were administered intravenously to aged CD1(ICR) mice (Charles River Laboratories) twice daily, once a week for three weeks (day 1, day 8 days and 15 days), followed by a 10-week no-treatment period. The test antibody was a commercially available mouse anti-glycation end product antibody raised against carboxymethyllysine conjugated to keyhole limpet hemocyanin, the carboxymethyllysine MAb (clone 318003) available from Available from R&D Systems, Inc. (Minneapolis, MN; Cat. No. MAB3247). A control reference of saline was used in control animals.
[0143...
Embodiment 2
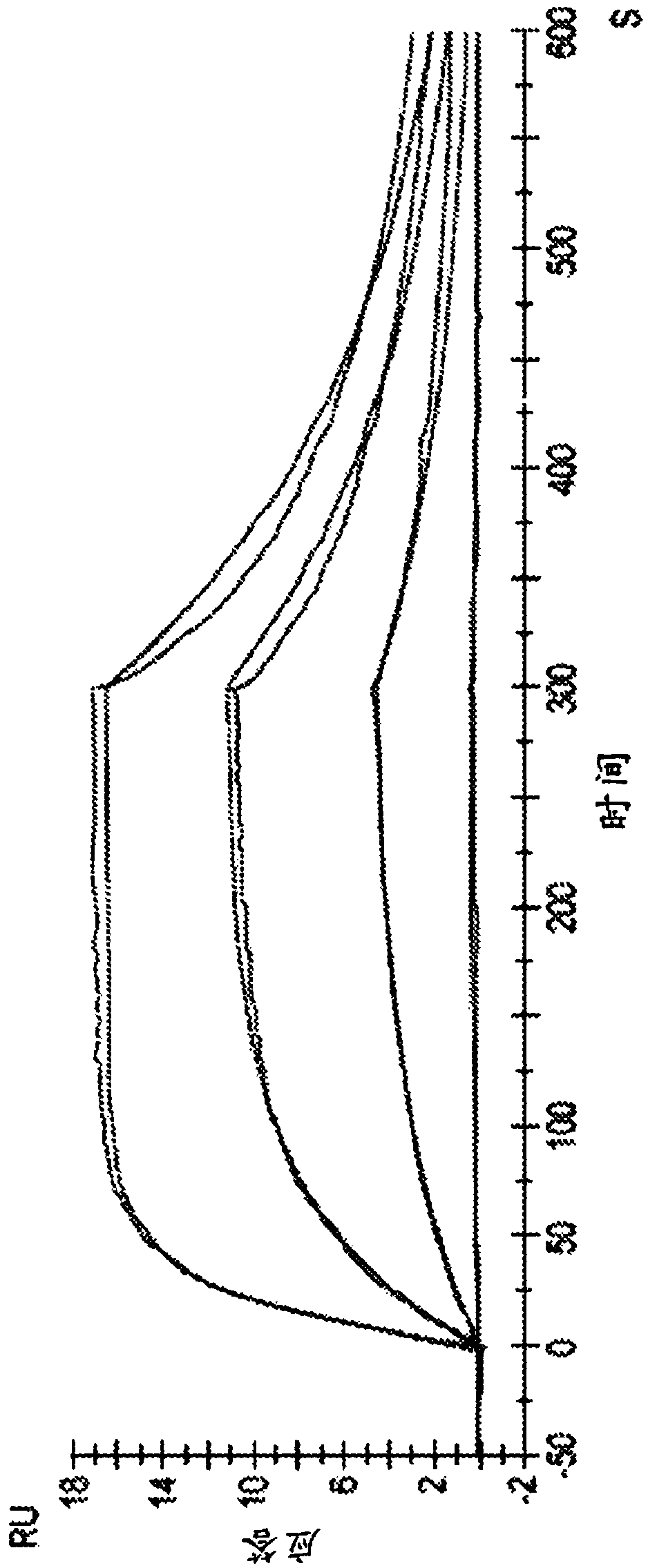
[0158] Example 2: Testing Antibody Affinity and Kinetics
[0159] Use Nα, Nα-bis(carboxymethyl)-L-lysine trifluoroacetate (Sigma-Aldrich, St.Louis, MO) as a model substrate for the AGE-modified protein of cells used in Example 1 The affinity and kinetics of the test antibodies were analyzed. Using the series S sensor chip CM5 (GE Healthcare, Pittsburgh, PA) at BIACORE TM Label-free interaction assays were performed on a T200 (GE Healthcare, Pittsburgh, PA) with Fc1 set as blank and Fc2 immobilized with a test antibody (molecular weight 150,000 Da). The running buffer was HBS-EP buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA and 0.05% P-20, pH 7.4) at a temperature of 25°C. The software is BIACORE TM T200 Evaluation Software, Version 2.0. Dual references (Fc2-1 and buffer injection only) were used in the analysis and the data were fitted to a Langmuir 1:1 binding model.
[0160] Table 4: Experimental setup for affinity and kinetic analysis
[0161]
[0162] A plot of re...
Embodiment 3
[0163] Example 3: Construction and Production of Mouse Anti-AGE IgG2b Antibody and Chimeric Anti-AGE IgG1 Antibody
[0164] Murine and chimeric human anti-AGE antibodies were prepared. The DNA sequence of the murine anti-AGE antibody IgG2b heavy chain is shown in SEQ ID NO:12. The DNA sequence of the chimeric human anti-AGE antibody IgGl heavy chain is shown in SEQ ID NO:13. The DNA sequence of the murine anti-AGE antibody kappa light chain is shown in SEQ ID NO:14. The DNA sequence of the chimeric human anti-AGE antibody kappa light chain is shown in SEQ ID NO:15. The gene sequence was synthesized and cloned into a high expression mammalian vector. The sequence was codon optimized. Sequence confirmation of completed constructs was performed prior to proceeding with transfection.
[0165] HEK293 cells were seeded in shake flasks one day before transfection and grown using serum-free chemically defined media. The DNA expression constructs were transiently transfected into...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com