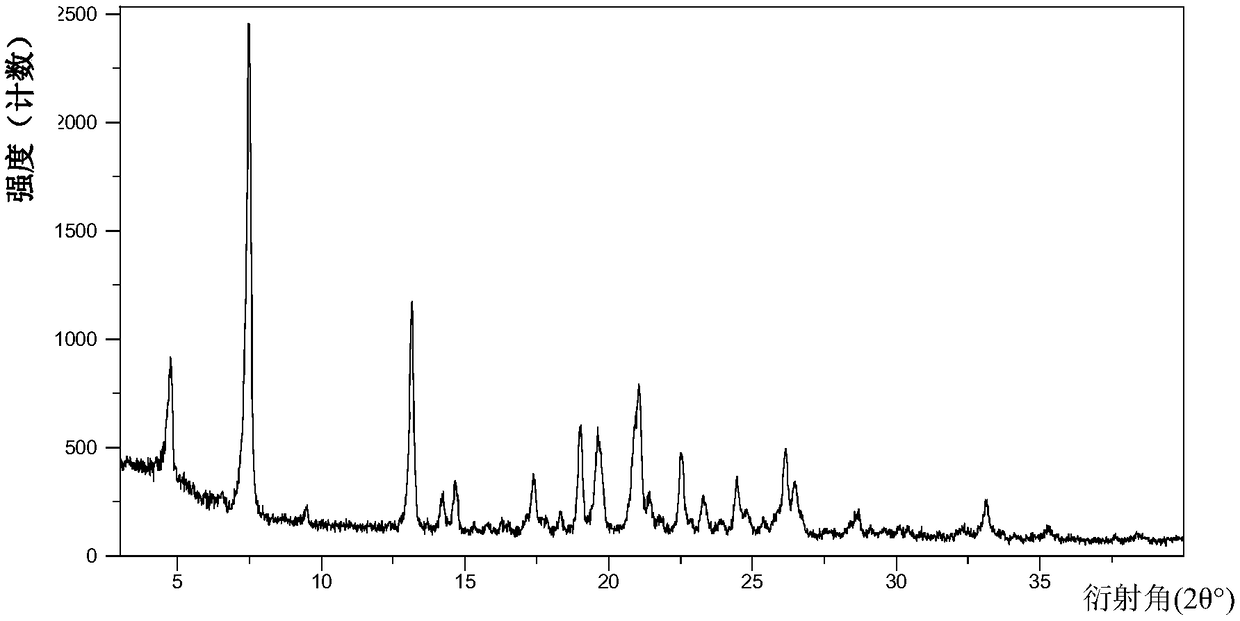
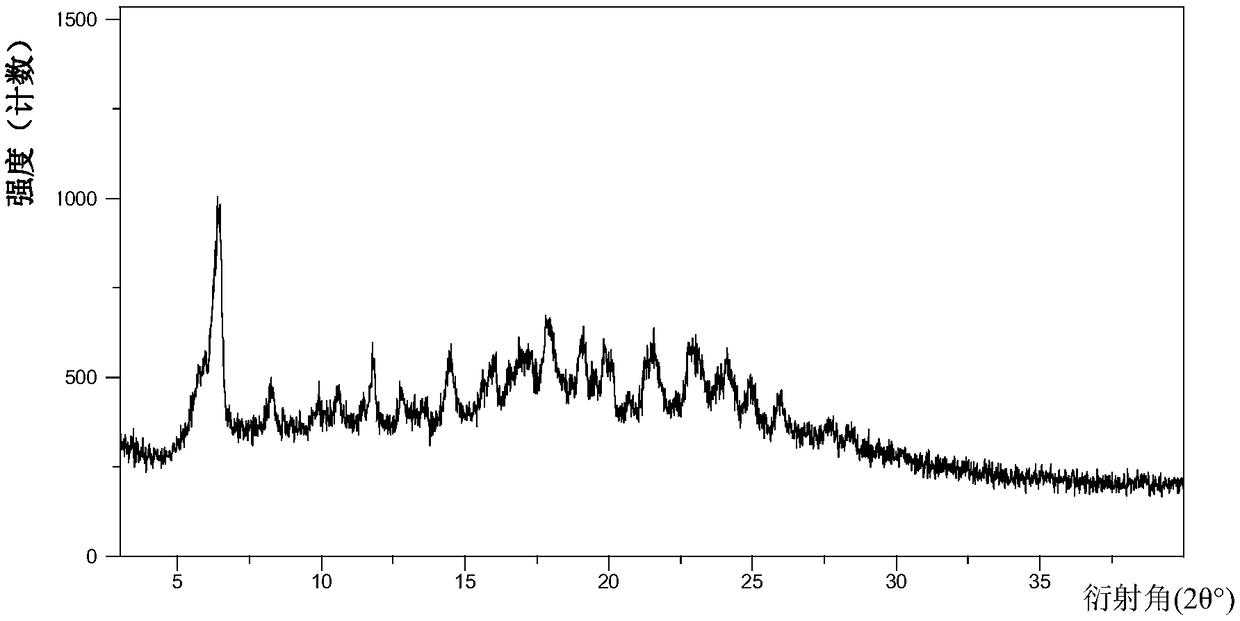
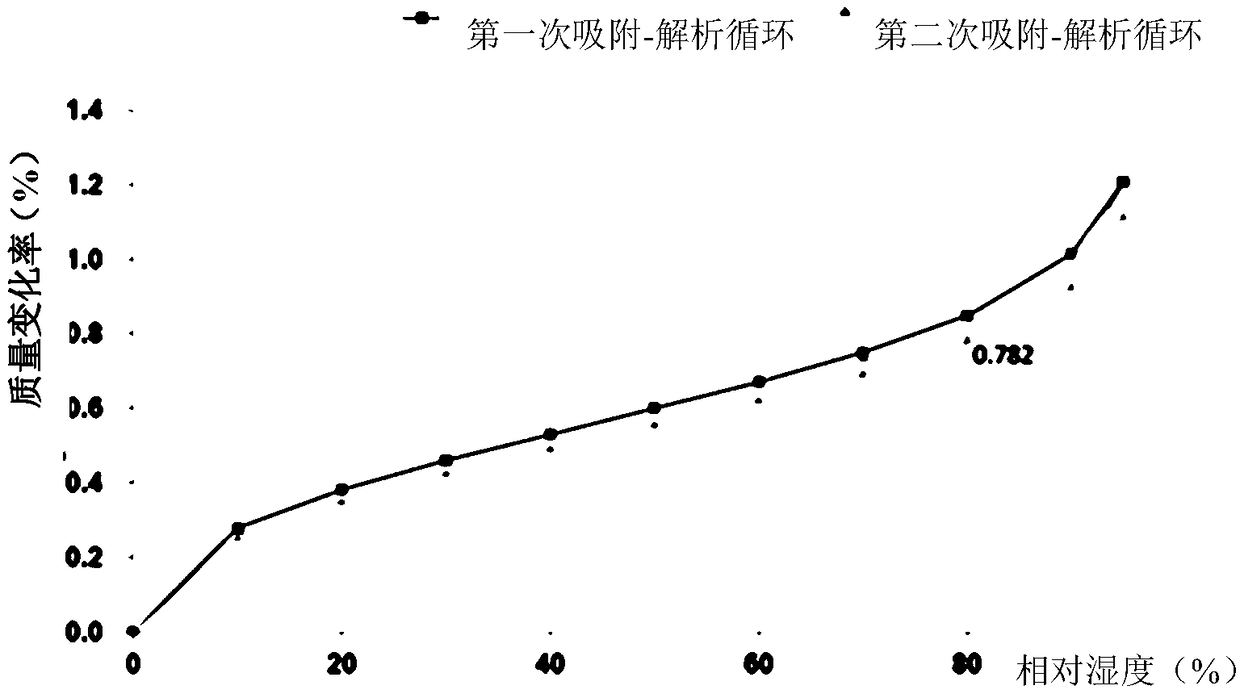
Protein tyrosine kinase modulators salt, crystallographic forms, and uses thereof
A technology of crystal form and use, applied in the field of composition, can solve problems such as infeasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] The preparation of embodiment 1 crystal form A
[0148] The free base was prepared by the method described in Example 74 of WO2015 / 003658.
[0149] 350 mg of free base and 70.1 mg of succinic acid were added to a 20 ml glass vial, then 10 ml of EtOAc was added to the glass vial. The mixture was stirred at RT for about 24 hours, then the wet cake was filtered and dried at 50 °C under reduced pressure to afford 314 mg of Form A. MS: m / z 710.3, mp: about 142.0°C. .
[0150] 1 H NMR (400MHz,DMSO)δ=11.36(s,1H),9.03(s,1H),8.83(s,1H),8.63(s,1H),8.26(s,1H),8.08(s,1H) ,7.63(d,J=6.7,1H),7.52(d,J=8.2,1H),7.15(t,J=7.5,1H),6.88(s,1H),6.61(dd,J=17.0,10.2 ,1H),6.17(d,J=17.0,1H),5.71(d,J=10.9,1H),3.78(s,4H),3.01(s,4H),2.90(s,8H),2.61(s ,4H), 2.41(s,5H), 2.30(s,3H), 0.85(d,J=6.6,0H).
Embodiment 2
[0151] The preparation of embodiment 2 crystal form A
[0152] The free base was prepared by the method described in Example 74 of WO2015 / 003658.
[0153] 350 mg free base and 70.1 mg succinic acid were added to a 100 ml glass vial, then 20 ml EtOAc was added to the glass vial. The mixture was stirred at RT for about 96 hours, then the wet cake was filtered and dried at 50 °C under reduced pressure to yield 317 mg of Form A. MS: m / z 710.3, mp: about 141.0°C-143.0°C.
Embodiment 3
[0154] The preparation of embodiment 3 crystal form A
[0155] The free base was prepared by the method described in Example 74 of WO2015 / 003658.
[0156] About 340.1 g of free base and 71.1 g of succinic acid were charged to the reactor, and then 27.2 L of acetone was added to the reactor. The mixture was stirred at 50°C for about 8 hours, cooled to room temperature, then the wet cake was filtered and dried under reduced pressure at 50°C to obtain 285.6 g of Form A. MS: m / z 710.3, mp: about 141.0°C-143.0°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com