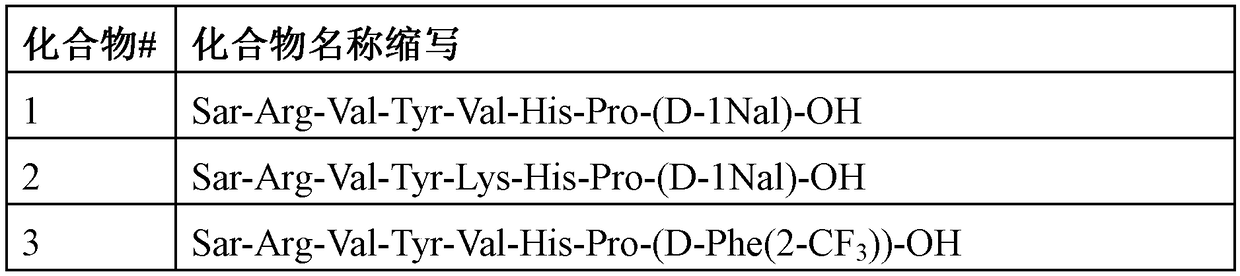
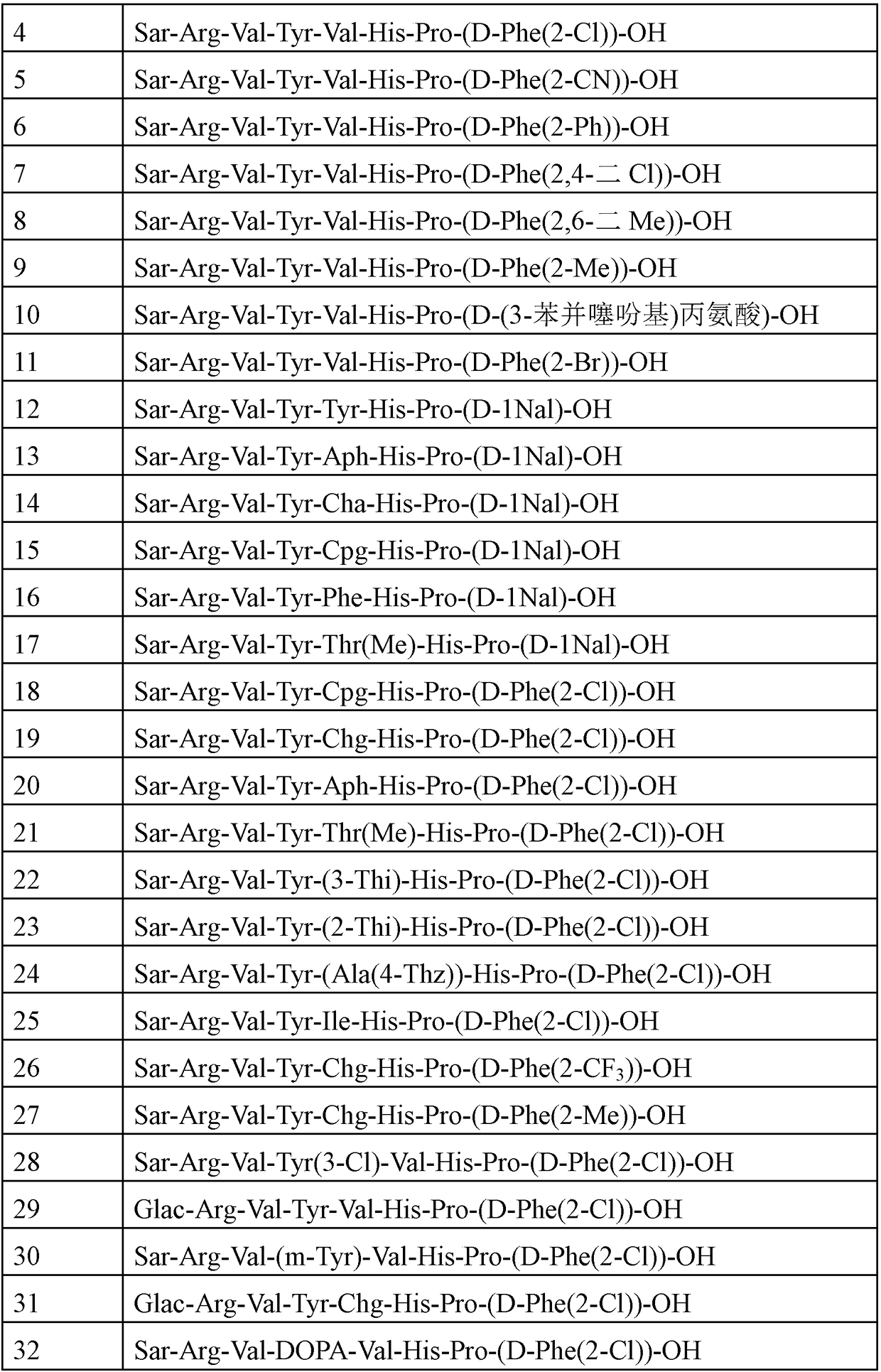
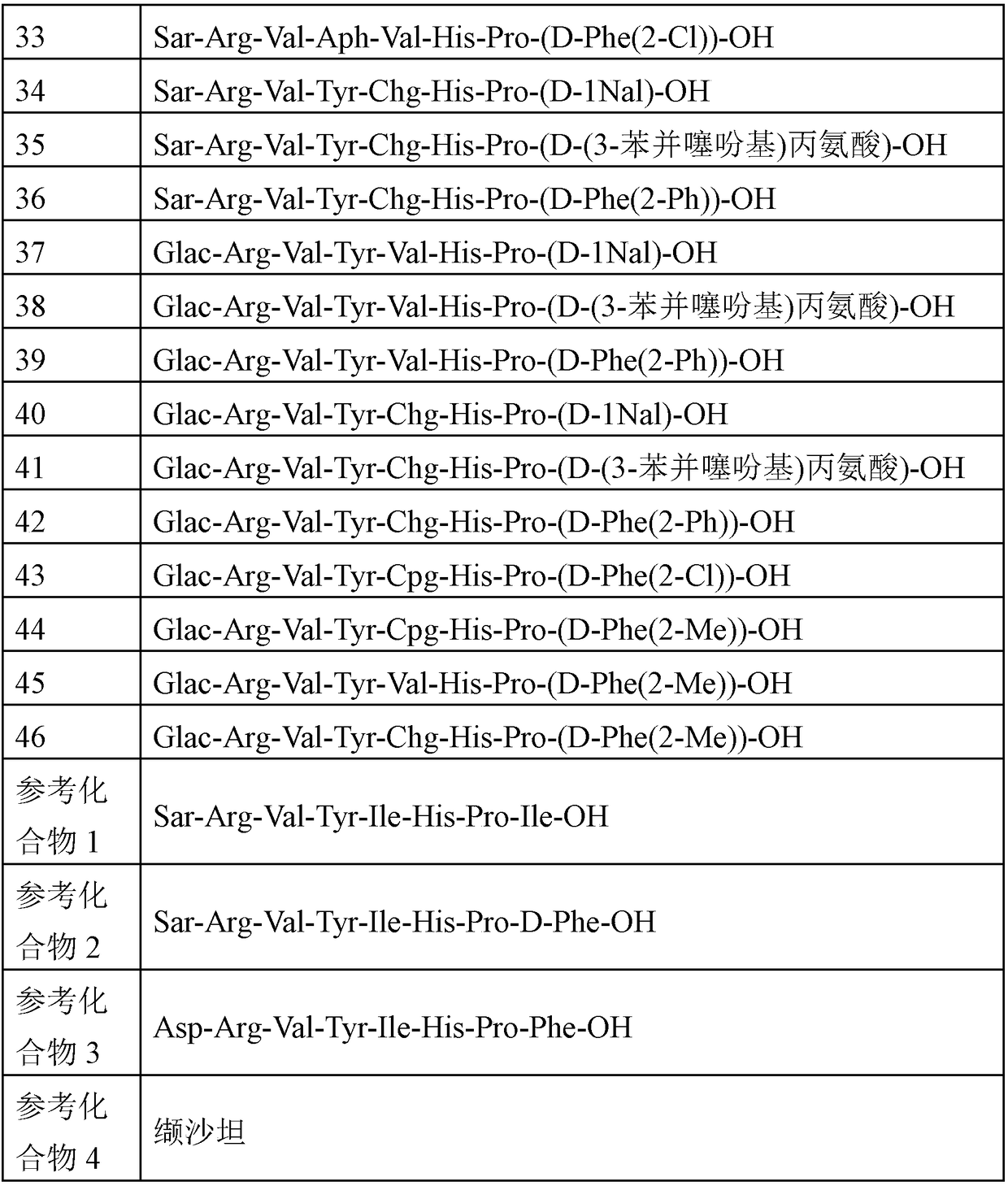
Angiotensin-1-receptor antagonists
A compound, free technology, applied in angiotensin, cardiovascular system diseases, peptide/protein components, etc., can solve problems such as lack of treatment options for hypertension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The preparation of the AT1R antagonist peptides described herein may involve the protection and deprotection of various chemical groups. One skilled in the art can readily determine the need for protection and deprotection and the selection of appropriate protecting groups. The chemistry of protecting groups can be found, for example, in T.W. Greene and P.G.M. Wuts, Protective Groups in Organic Synthesis, 3rd Ed., Wiley & Sons, Inc., New York (1999), which is hereby incorporated by reference in its entirety.
[0050] The AT1R antagonist peptides described herein may also include all isotopes of atoms present in intermediate or final compounds. Isotopes include atoms with the same atomic number but different mass numbers. For example, isotopes of hydrogen include tritium and deuterium.
[0051] All compounds and their pharmaceutically acceptable salts may exist together with other substances such as water and solvents (eg, hydrates and solvates), or may be isolated.
...
Embodiment 1
[0089] Example 1: Synthesis of Compound 1.
[0090]The starting 2-chlorotrityl polystyrene resin (Peptides International, catalog number RCT-1083-PI, 1.39mmol / g), 1.87g, 2.6mmol) was mixed with Fmoc-D-1-Nal (1.3mmol ) and a solution of N,N-diisopropylethylamine (DIPEA, 6.5 mmol) in DCM (25 ml) were reacted for 2.5 hours. After addition of MeOH (2.5ml), the mixture was swirled for another 30 minutes. The reagents were drained and the resin was washed with DMF. The resin was divided between two 40ml reaction vessels (0.65mmol per vessel).
[0091] Solid-phase peptide synthesis was performed using a Tribute peptide synthesizer. Coupling of Fmoc-protected amino acids on a Tribute synthesizer was mediated with HBTU / NMM in DMF. A single cycle of 60 minutes was used during synthesis with 2.5 mmol of activated Fmoc-protected amino acid per vessel (3.8-fold excess). Arginine was coupled within 3 hours. The removal of the Fmoc protecting group was monitored by UV. The peptide re...
Embodiment 2
[0095] Embodiment 2: the synthesis of compound 4
[0096] Peptides were manually assembled starting from 13.158 g (20 mmol) of 2-chlorotrityl polystyrene resin (Peptides International, catalog number RCT-1083-PI, 1.52 mmol / g). A solution of Fmoc-D-Phe(2-Cl) (4.219 g, 10 mmol) and DIPEA (8.8 ml, 50 mmmol) in DCM (75 ml) was added to the dry resin. The reaction was carried out for 6 hours. After addition of methanol (8 ml), the mixture was stirred for 30 minutes, the reagents were drained and the resin was washed with DMF.
[0097] From this point, DIC / Oxyma Pure mediated coupling in NMP was employed. Single cycles of at least 2 hours and up to 24 hours were used during synthesis with up to a 2-fold excess of activated Fmoc-protected amino acids. Integrity of coupling was assessed using the ninhydrin test. Removal of the Fmoc protecting group was achieved by washing the peptide resin twice (5 min, then 20 min) with 20% piperidine in DMF.
[0098] First, the (6-8) fragment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com