New cedar pollen protein
A fir tree and pollen technology, applied in the field of new fir tree pollen protein, can solve the problem of ineffective allergen immunotherapy and achieve the effect of effective desensitization treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
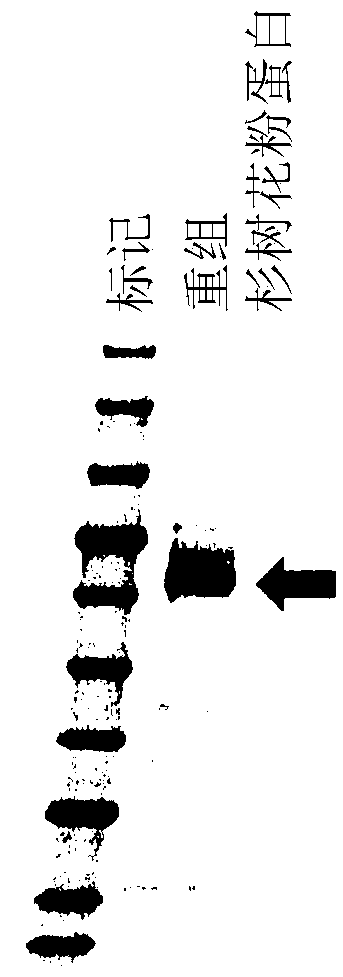
[0100] The rough refinement of embodiment 1 novel fir tree pollen protein
[0101]
[0102] 50 mL of an extraction buffer (20 mM Tris-HCl, pH 9.0) was added to 2 g of cedar tree pollen, and ultrasonication was performed for 10 minutes. Next, after mixing at room temperature for 10 minutes, it was centrifuged (10,000 g, 10 minutes) to obtain a supernatant. This supernatant was again subjected to ultracentrifugation (50,000 g, 60 minutes), and the supernatant was recovered. To 17 mL of this supernatant, an equal amount of Q sepharose high performance resin (GE Healthscare) suspension equilibrated with an extraction buffer (20 mM Tris-HCl, pH 9.0) was added, followed by shaking at room temperature for 30 minutes. After the supernatant was removed by centrifugation (1,000 g, 10 minutes), the volume was adjusted to 50 mL with extraction buffer (20 mM Tris-HCl, pH 9.0), mixed by inversion, and the resin was washed by centrifugation again. Next, 10 mL of an extraction buffer (20 ...
Embodiment 2
[0105] Embodiment 2 Preparation of refined recombinant protein
[0106]
[0107] 2 g of cedar tree male flowers (anthers) frozen and pulverized in liquid nitrogen were added to a 50 mL tube containing 25 mL of Plant RNA Isolation Reagent (Invitrogen), mixed, and left to stand at room temperature for 5 minutes.
[0108] After centrifugation at 4° C. (2600×g, 5 minutes), the upper layer was filtered through a mesh with a mesh size of 100 μm by decantation. Next, 1 / 5 times the amount of 5M NaCl and 3 / 5 times the amount of chloroform were added to the recovered filtrate. After centrifugation at 4° C. (2600×g, 30 minutes), 20 mL of the upper layer was recovered, and 9 / 10 times the amount of isopropanol was added and stirred. After standing still at room temperature for 10 minutes, the RNA was precipitated by centrifugation at 4°C (2600 x g, 30 minutes). After removing the supernatant, 10 mL of 75% ethanol was added to the RNA pellet for washing. Then, after centrifugation at 4...
Embodiment 3
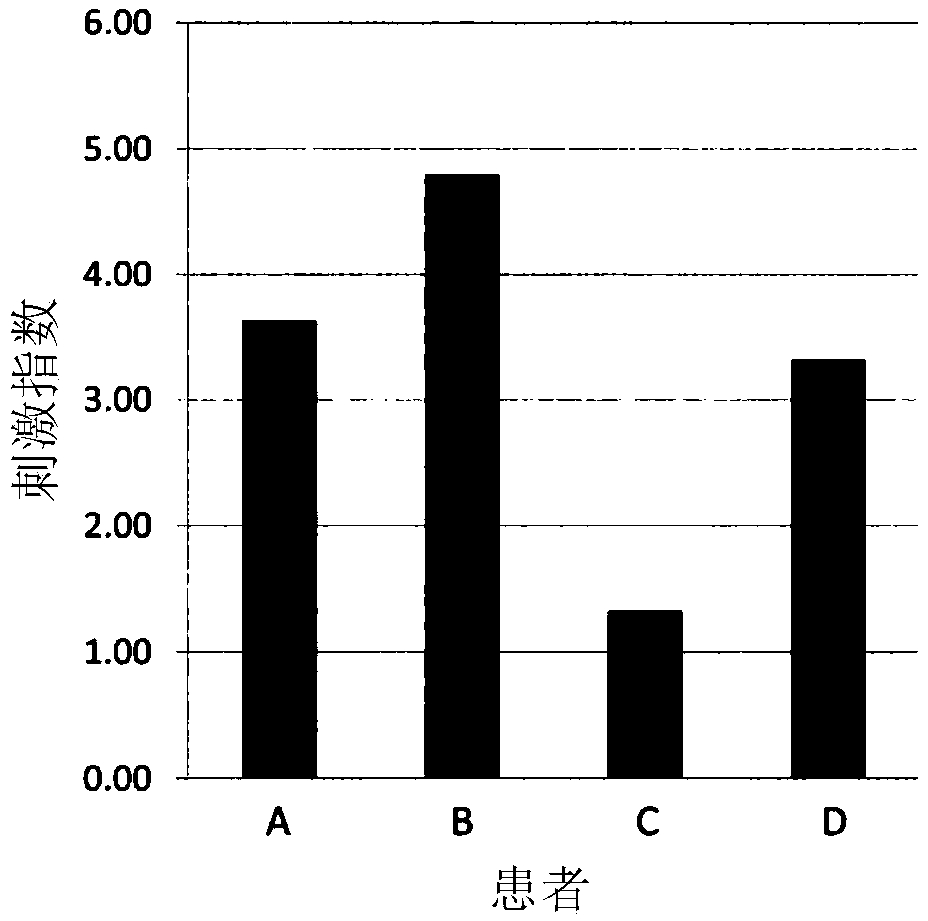
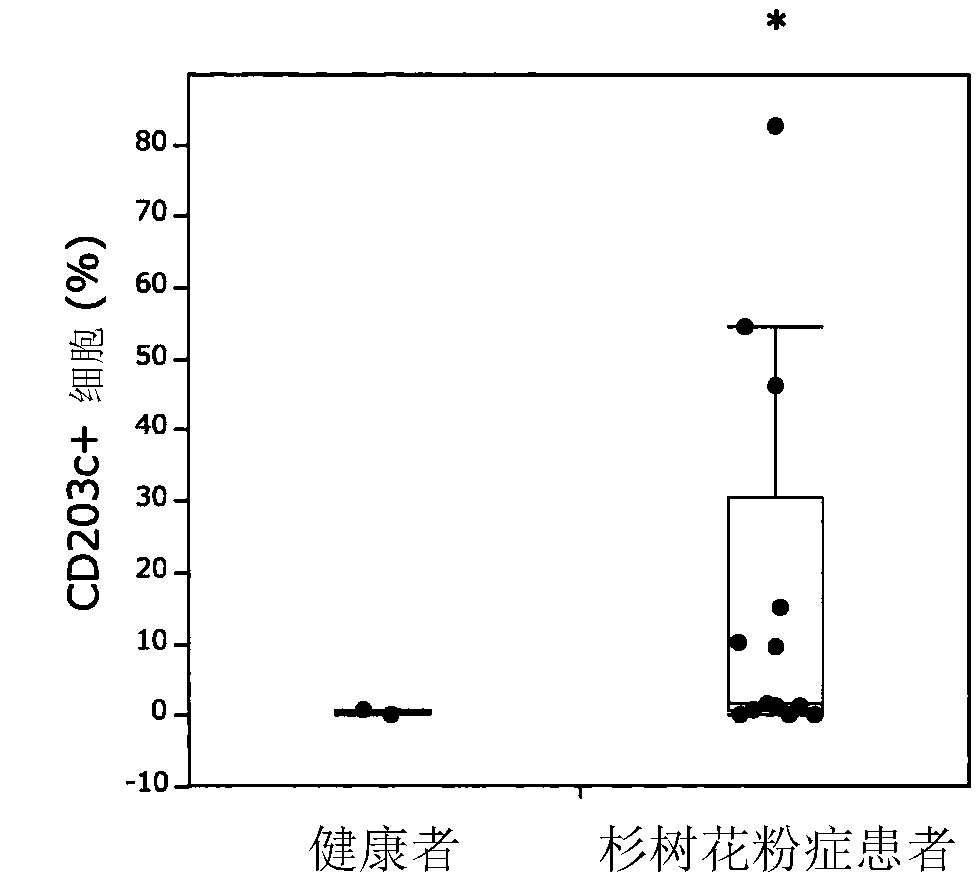
[0116] Example 3 Allergen Activity Confirmation of Refined Recombinant Protein
[0117] (i) Lymphocyte proliferation response in patients with cedar tree hay fever
[0118] Peripheral blood mononuclear cells were isolated from 30 mL of peripheral blood obtained from cedar hay fever patients (ImmunoCAP score ≥ 2) to become 1.11×10 6 cells / mL were suspended in RPMI-1640 medium containing 10% non-activated autologous plasma. Inoculate 180 μL of the prepared cell suspension and 20 μL of the recombinant protein solution (concentration 100 μg / mL) of the present invention on a 96-well plate (2×10 5 cells / well). At 37°C, 5% CO 2 After culturing for 3 days under the condition, add 3 For H-thymidine, the uptake amount after 16 hours was measured as radioactivity, thereby evaluating cell proliferation. Calculate the time when adding purified recombinant protein 3 The value obtained by dividing the intake of H-thymidine by the intake when it was not added was used as a stimulation i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com