Preparation method of 2,4,6-trimethylbenzoic acid
A technology of trimethylbenzoic acid and mesitylene, applied in the direction of carboxylate preparation, organic compound preparation, chemical instruments and methods, etc., can solve the problem of low purity of trimethylbenzoic acid and achieve the effect of continuous reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
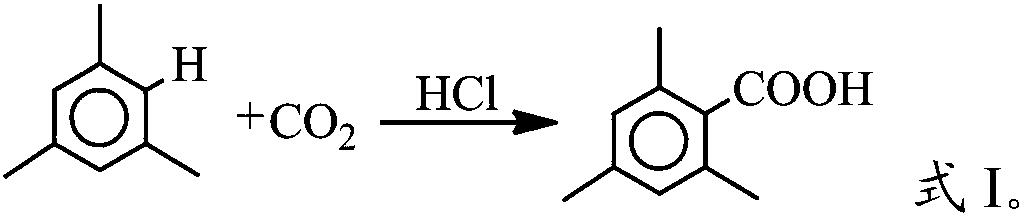
[0019] The invention provides a kind of preparation method of 2,4,6-trimethylbenzoic acid, comprising the following steps:
[0020] Reaction of mesitylene and carbon dioxide under the action of a catalyst to obtain 2,4,6-trimethylbenzoic acid; the catalyst is hydrogen chloride or concentrated hydrochloric acid.
[0021] In the present invention, unless otherwise specified, all raw materials are commercially available goods.
[0022] In the invention, mesitylene and carbon dioxide are reacted under the action of a catalyst to obtain 2,4,6-trimethylbenzoic acid.
[0023] In the present invention, the carbon dioxide is preferably liquid carbon dioxide. In the present invention, the catalyst is hydrogen chloride or concentrated hydrochloric acid; when the catalyst is concentrated hydrochloric acid, the mass concentration of the concentrated hydrochloric acid is preferably 37%-40%, more preferably 38%-39%. The mass of the catalyst is 0.1%-20% of the mass of mesitylene, more prefe...
Embodiment 1
[0037] Add various reaction raw materials into the material tank, control the flow of mesitylene to 50mL / min, the temperature in the microreactor to 70°C, the pressure to 3.0MPa, the molar ratio of mesitylene to carbon dioxide is 1:0.5, and the catalyst is a mass concentration of 38%. Concentrated hydrochloric acid, use the length of the coil to control the reaction time to 25s, separate layers after the reaction, the lower layer concentrated hydrochloric acid is recovered, the upper layer is cooled and centrifugally filtered, the solid is collected to obtain 2,4,6-trimethylbenzoic acid, and the filtered filtrate is transported Return to the material tank to continue the reaction, and the product has a purity of 99.1%.
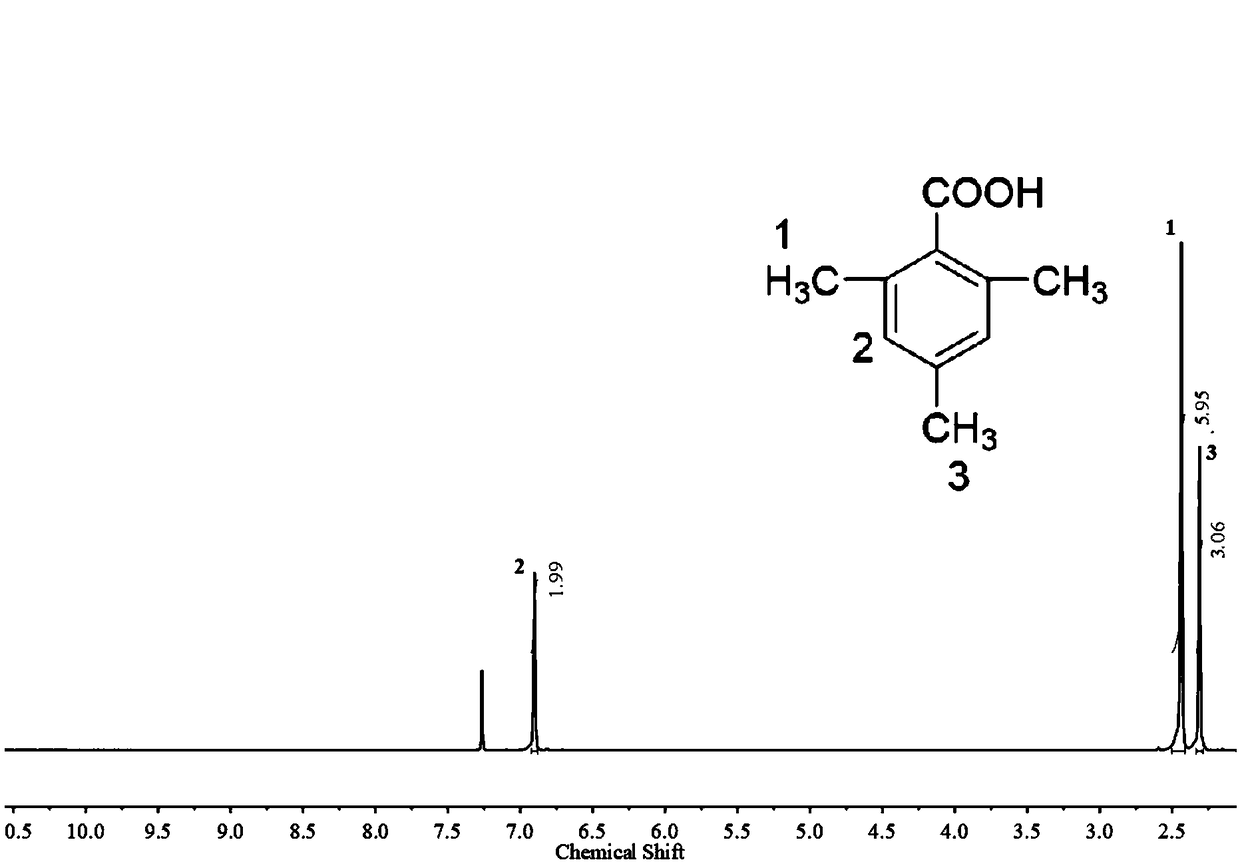
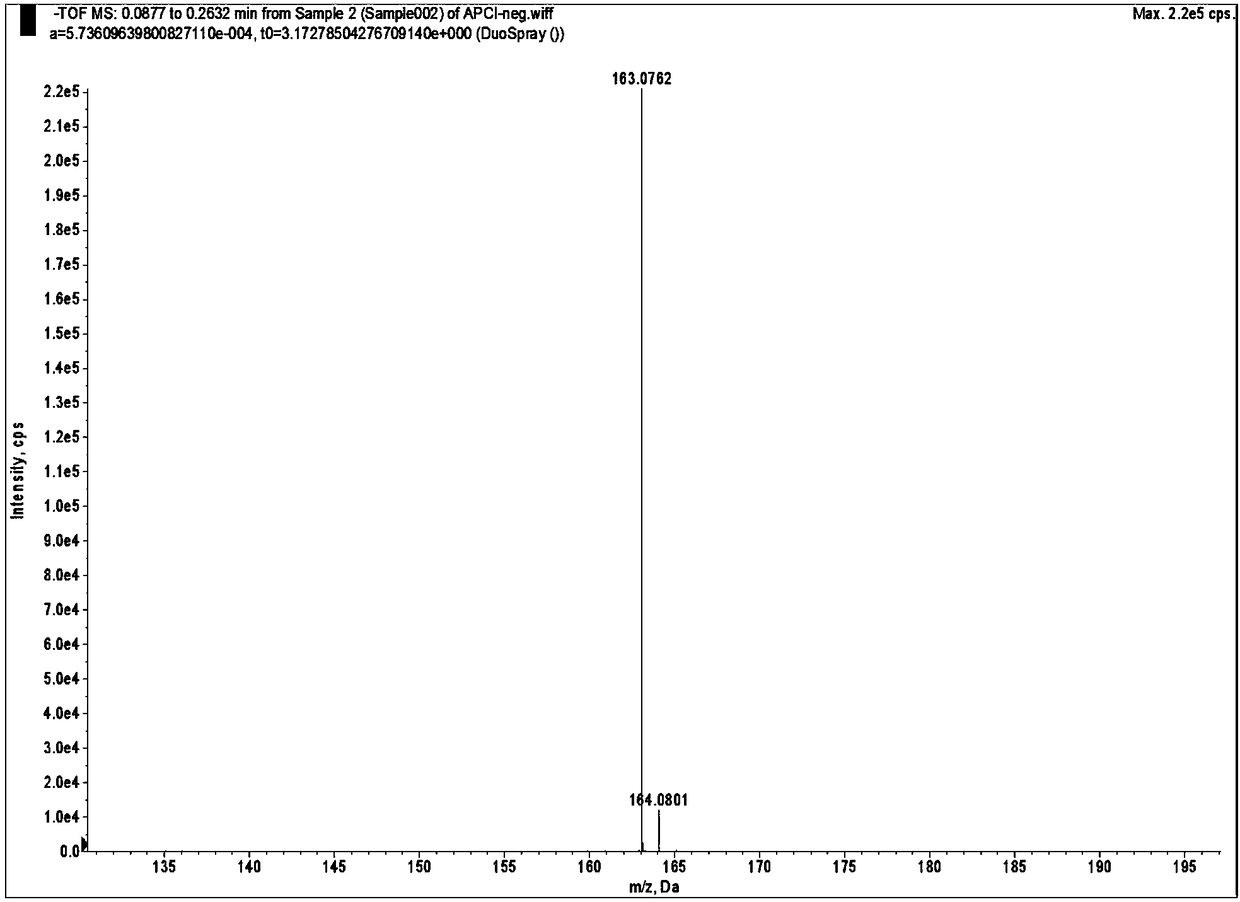
[0038] Carry out nuclear magnetic analysis and mass spectrometry to the 2,4,6-trimethylbenzoic acid that embodiment 1 prepares, analysis result is as follows figure 1 with figure 2 shown, where figure 1 It is the NMR spectrum of 2,4,6-trimethylbenzoic acid,...
Embodiment 2
[0040] Add various reaction raw materials into the material tank, control the flow rate of mesitylene to 100mL / min, the temperature in the microreactor to 40°C, the pressure to 3.5MPa, the molar ratio of mesitylene to carbon dioxide is 1:0.3, and the catalyst is hydrogen chloride gas. The length of the tube is controlled and the reaction time is 25s. After the reaction is completed, the solid is collected by cooling and centrifugal filtration to obtain 2,4,6-trimethylbenzoic acid. The filtered filtrate is sent back to the material tank to continue the reaction. The product purity is 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com