Preparation method of dapoxetine hydrochloride free alkali
A compound and oily technology, which is applied in the field of preparation of dapoxetine hydrochloride free base, can solve the problems of poor stability of liquid products, unfavorable development of solid preparations and unfavorable storage of liquid free base, and achieve high yield, convenient mass production, The effect of good practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The present invention includes a preparation method of the compound of formula I, wherein the preparation is an improvement over the synthesis method of the existing patented dapoxetine hydrochloride free base I.
[0022] definition:
[0023] As used herein, the term "contacting" refers to bringing two or more chemical molecules into proximity so that the two or more chemical molecules react. For example, partially or completely dissolving two or more chemical molecules in one or more solvent systems, mixing one or more chemical substances in a solvent with another or more solid or gas phase chemical substances, etc. Other methods generally known to those skilled in the art.
[0024] As used herein, the term "reaction conditions" refers to the specific circumstances under which a chemical reaction takes place. Including (but not limited to) one or more of the following factors: temperature, solvent, pH, pressure, reaction time, mole ratio of reactants, catalyst, etc. ...
Embodiment 1
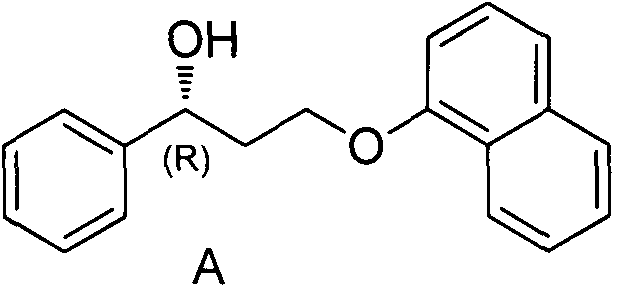
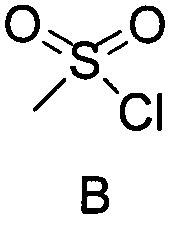
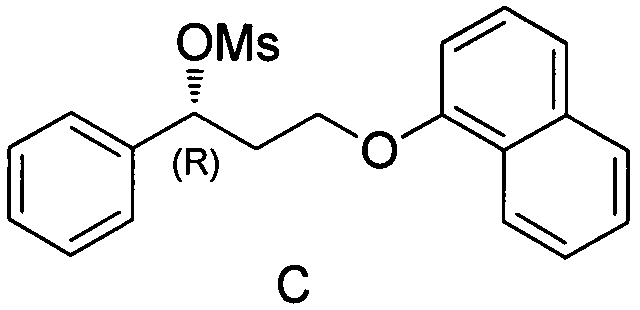
[0056] The first step: the compound of formula A and the compound of formula B generate the compound of formula C
[0057] In a 250mL three-necked round-bottomed flask equipped with a magnetic stirrer, add 20ml of methyl tert-butyl ether, add 20mmol of compound A, 40mmol of triethylamine in turn, start stirring, control the temperature at 5-15°C, keep stirring until dissolved After clearing, add 22 mmol methanesulfonyl chloride dropwise, stir for 1 h, and take a sample. TLC detects that the reaction of compound A is basically complete, and the temperature is controlled at 5-15°C for the next step of reaction.
[0058] The second step: the compound of formula C and the compound of formula D generate the compound of formula I
[0059] In the round-bottomed flask in the first step, the temperature was controlled at 5-15 ° C, and 140 mmol of compound D in 2M dimethylamine tetrahydrofuran solution was added, and the temperature was raised to 35 ° C, and stirred until the reaction o...
Embodiment 2
[0068] The first step: the compound of formula A and the compound of formula B generate the compound of formula C
[0069] In a 250mL three-necked round-bottomed flask equipped with a magnetic stirrer, add 20ml of methyl tert-butyl ether, add 20mmol of compound A, 40mmol of triethylamine in turn, start stirring, control the temperature at 5-15°C, keep stirring until dissolved After clearing, add 22 mmol methanesulfonyl chloride dropwise, stir for 1 h, and take a sample. TLC detects that the reaction of compound A is basically complete, and the temperature is controlled at 5-15°C for the next step of reaction.
[0070] The second step: the compound of formula C and the compound of formula D generate the compound of formula I
[0071] In the round-bottomed flask in the first step, the temperature was controlled at 5-15 ° C, and 140 mmol of compound D in 2M dimethylamine tetrahydrofuran solution was added, and the temperature was raised to 35 ° C, and stirred until the reaction o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com