Treatment of retinal vascular disease using progenitor cells
A technology for retinopathy, the retina, applied in the field of cell-based or regenerative therapy, which can solve the problem of destroying healthy tissue and no treatment for dry AMD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] cell preparation
[0069] Cells, cell populations, and preparations (including cell lysates, conditioned media, etc.) for use in the compositions and methods of the invention are described herein and detailed in U.S. Patent Nos. 7,524,489 and 7,510,873 and U.S. Published Application 2005 / 0058631, each of which is incorporated herein by reference. According to the method using postpartum cells, the mammalian umbilical cord or placenta is recovered at or shortly after the end of a term or preterm pregnancy (eg, after expulsion of the placenta). Postpartum tissue can be transported from the delivery site to the laboratory in sterile containers such as flasks, beakers, petri dishes, or bags. The container may have a solution or medium, including but not limited to, for example, a saline solution such as Dulbecco's Modified Eagle's Medium (DMEM) or Phosphate Buffered Saline (PBS), or any solution used to transport organs for transplantation, such as University of Wiscon...
Embodiment 1
[0163] hUTC efficacy in the rat OIR model
[0164] To study the effect of hUTC on retinal neovascularization.
[0165] Materials and methods
[0166] Human umbilical cord tissue-derived cells (hUTC) are described in Examples 4-16 below and detailed in U.S. Pat. method to obtain. Briefly, human umbilical cords were obtained with the consent of the donor after a live birth. Tissues were minced and digested enzymatically. Using 0.5U / mL collagenase (Serva Elecrtrophoresis, Heidelberg, Germany), 5U / mL neutral protease (Serva Elecrtrophoresis, Heidelberg, Germany) and 2U / mL hyaluronidase (Cumulase; Origio a / s, Denmark) of the mixture of Dalbecco's Modified Eagle's Medium (DMEM)-Low Glucose (Lg) (SAFC Biosciences, Lenexa, KS) after almost complete digestion, the cell suspension was filtered through a 70 μm filter membrane and incubated at 250× g Next centrifuge the supernatant. The detached cells were washed several times in DMEM-Lg and then washed at 5,000 cells / cm 2 was...
Embodiment 2
[0197] Efficacy of hUTC via subretinal injection in the rat OIR model
[0198] To study the effect of subretinal injection of hUTC on retinal neovascularization.
[0199] Materials and methods
[0200] hUTC were obtained as described above in Example 1.
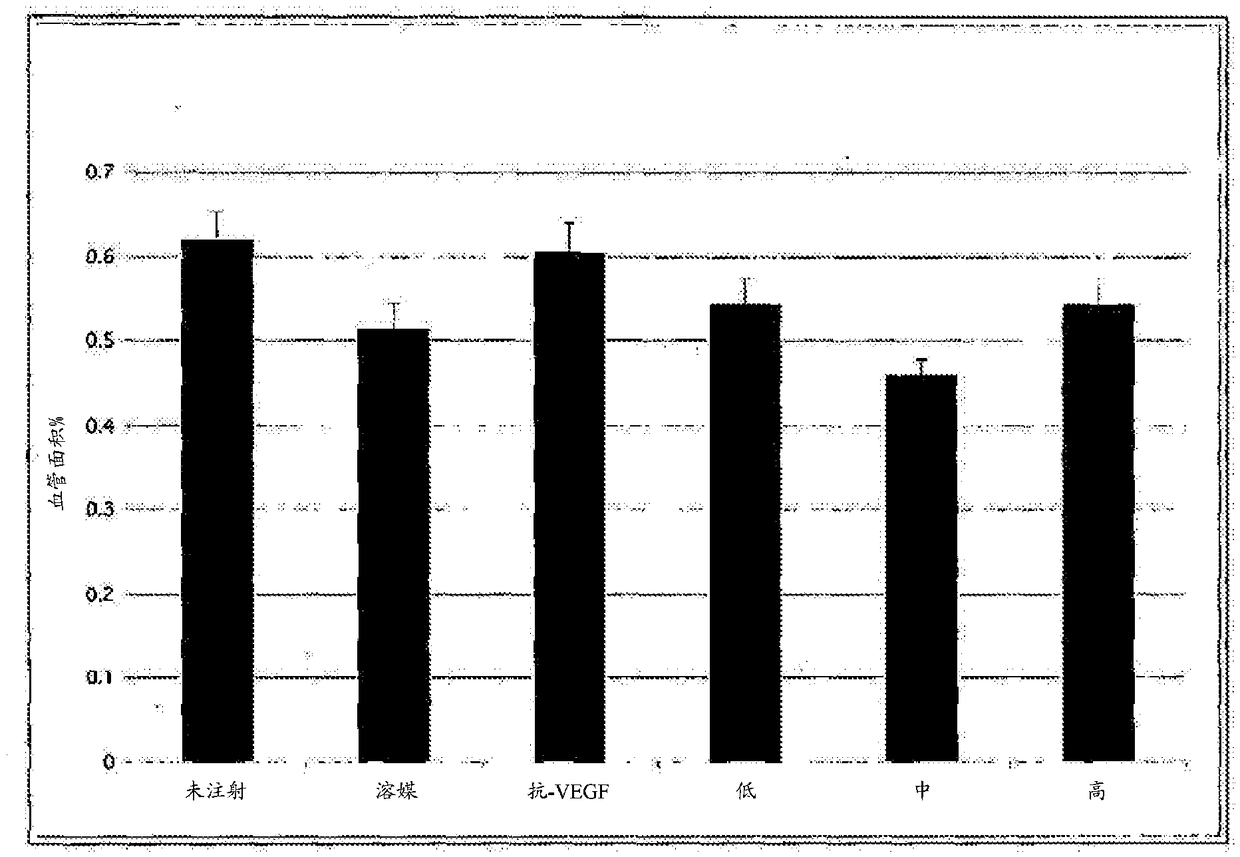
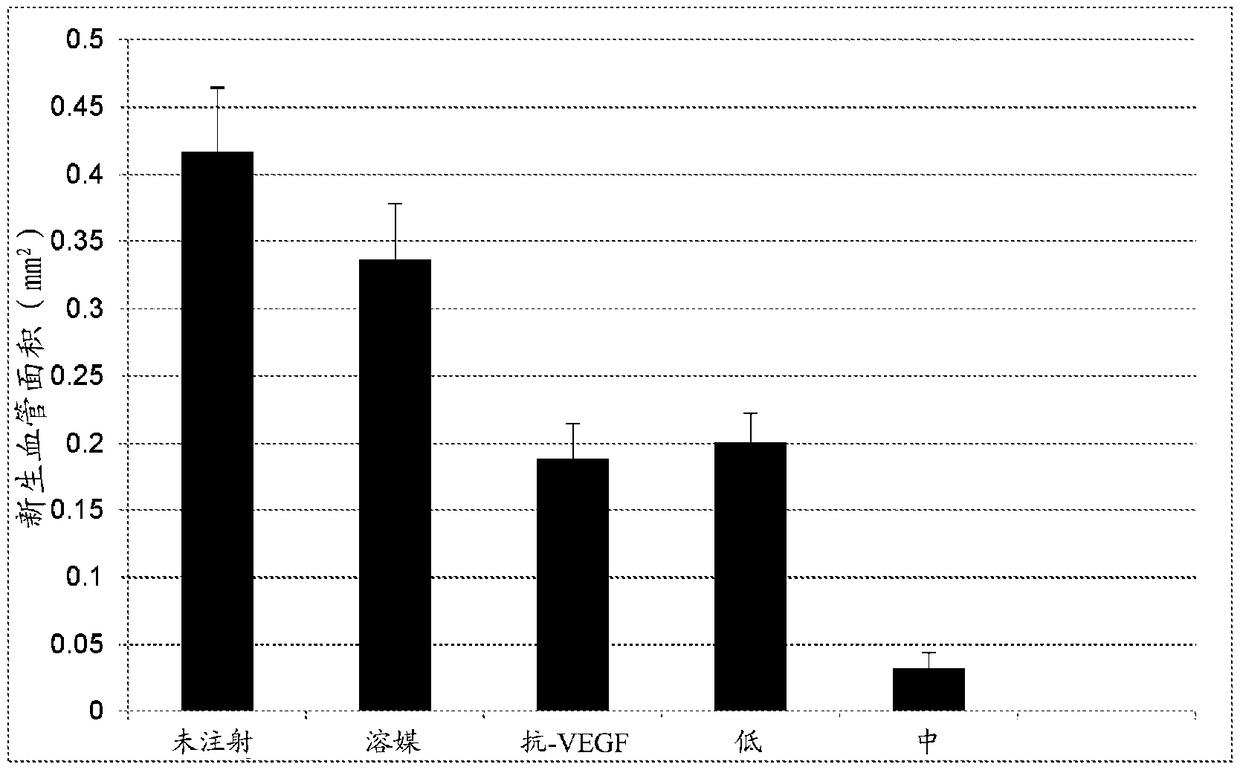
[0201] Sprague Dawley rats were housed from birth to P14 in a variable oxygen atmosphere consisting of 24 h alternating 50% and 10% oxygen events. Upon removal from the oxygen exposure chamber (P14), rats received subretinal injections at two doses / density (4×10 3 or 2×10 4 cells), or vehicle (cryopreservation solution). The treatment group distribution is shown in Table 2-1 below. All pups were sacrificed on day six post-exposure / injection (P20). Pup eyes were enucleated and normal intraretinal vascular growth and abnormal preretinal neovascular growth were assessed on ADPase-stained retinal flat mounts using widely published methods. The area of normal and abnormal vessel growth was measured via computer-aided ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com