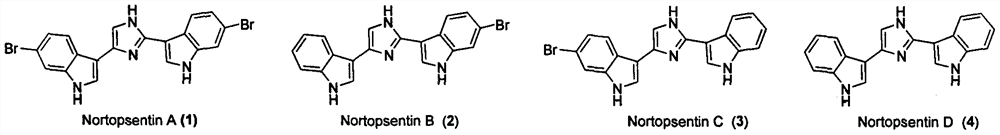
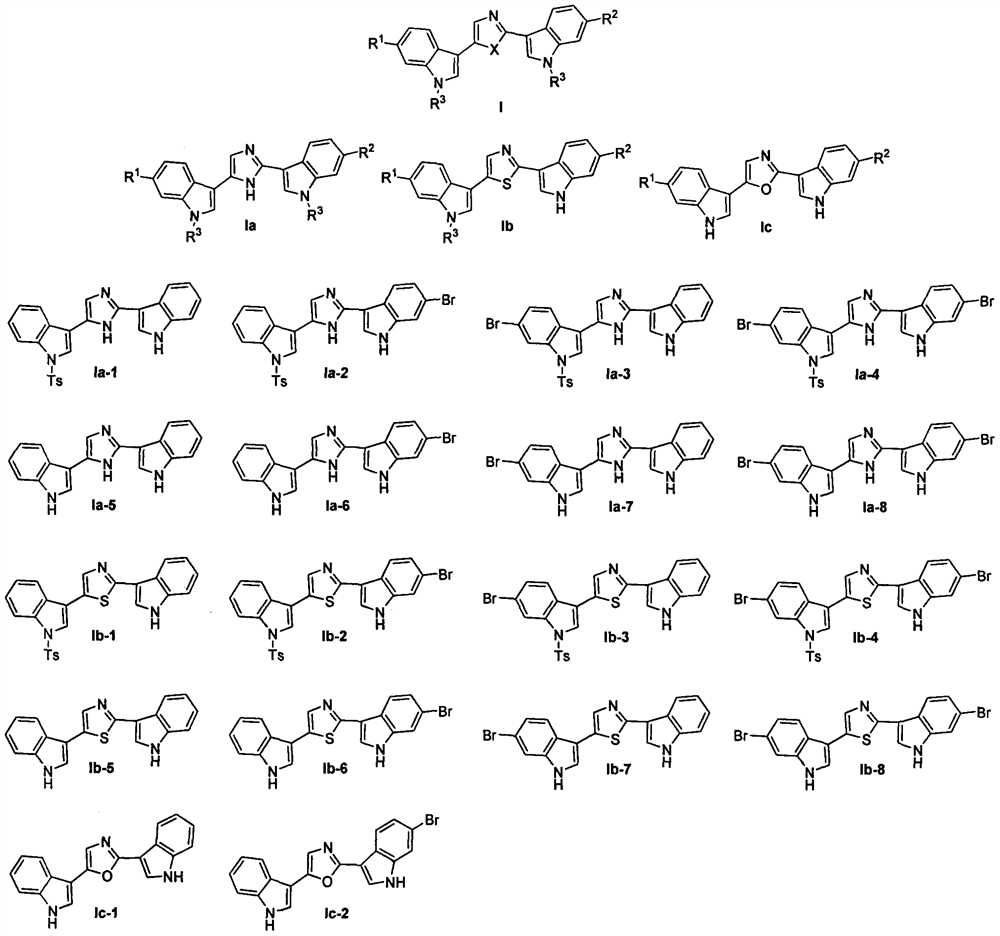
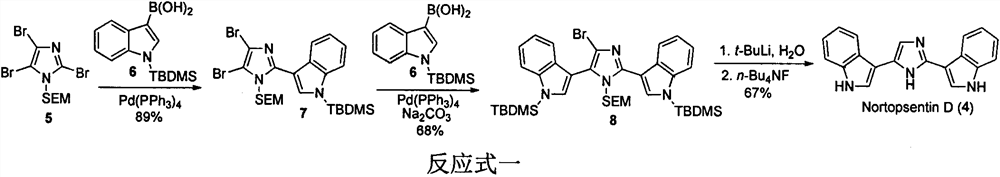
Application of nortopsentin alkaloids and their derivatives in the control of plant diseases and insect pests
A technology of alkaloid derivatives and derivatives, applied in the fields of chemicals, applications, biocides for biological control, etc., can solve the problems of difficult synthesis, low natural content of Nortopsentin alkaloids, and insufficient research on biological activities. , to achieve the effect of good insecticidal activity, good anti-plant virus and fungus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Synthesis of Nortopsentin alkaloid bisindole derivative structure Ia
[0031]
[0032] 18-1: Add indole (3.51g, 30mmol) and 150mL acetonitrile to a 500mL single-necked bottle in sequence, add 60% NaH (1.44g, 42mmol) under ice-cooling, stir for about 10min, add p-toluenesulfonyl chloride (6.27mmol) in batches g, 33mmol), after adding, return to room temperature reaction. The reaction was monitored by TLC, and the reaction was completed in about 4 hours. with saturated NH 4 The Cl solution was quenched, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and precipitated to obtain 8.06 g of a brown solid with a yield of 99% and a melting point of 76-78°C. 1 H NMR (400MHz, CDCl 3 ) δ7.99(d, J=8.4Hz, 1H), 7.76(d, J=8.4Hz, 2H), 7.56(d, J=3.6Hz, 1H), 7.52(d, J=7.6Hz, 1H) , 7.36-7.27(m, 1H), 7.24-7.18(m, 3H), 6.65(d, J=3.6Hz, 1H).
[0033] 18-2: The operation is the same as 18-1 to obtain a brown solid with a yield of 99% and a melting point ...
Embodiment 2
[0053] Embodiment 2: Synthesis of Nortopsentin alkaloid bisindole derivative structure Ib
[0054]
[0055] 25-1: Take a 250mL single-necked bottle, dissolve 70% NaSH (1.60g, 20mmol) in 20mL DMF, add MgCl 2 ·6H 2 O (2.03g, 10mmol), 3-cyanindole (1.42g, 10mmol) was added in batches under stirring, monitored by TLC, and stirred at room temperature for about 90min to complete the reaction. Pour the green suspension into 100 mL of water, filter with diatomaceous earth, add 1N HCl until no solid precipitates, add ethyl acetate for extraction, dry with anhydrous sodium sulfate, and obtain 1.57 g of yellow solid after precipitation, yield 89% , melting point: 141-142°C. 1 H NMR (400MHz, DMSO-d 6 )δ11.78(s, 1H), 8.96(s, 1H), 8.82(s, 1H), 8.71-8.54(m, 1H), 8.09(d, J=2.8Hz, 1H), 7.49-7.40(m , 1H), 7.21-7.09(m, 2H). 13 C NMR (100 MHz, DMSO-d 6 )δ193.6, 136.8, 128.0, 1259, 122.0, 121.8, 120.7, 116.3, 111.9.
[0056] 25-2: Same operation as 25-1, yellow solid, yield 87%, melting ...
Embodiment 3
[0065] Embodiment 3: Synthesis of Nortopsentin alkaloid bisindole derivative structure Ic
[0066]
[0067] 26-1: Add 2.34g (20mmol) indole and 80mL anhydrous ether to a 250mL round-bottomed flask, add a solution of oxalyl chloride (26mmol) in ether dropwise at 0°C, react at 0°C for about 1.5h, monitor by TLC, the reaction After completion, it was directly sucked and washed with ice anhydrous ether to obtain 3.65 g of yellow powder with a yield of 89%. No need to process, directly feed the next reaction.
[0068] 26-2: same operation as 26-1, brownish yellow powder, yield 87%,
[0069] 27-1: Add compound 26-1 (0.21g, 1mmol) into a 100mL single-necked bottle, add 10mL of anhydrous diethyl ether, add 2mL of concentrated ammonia water dropwise and stir at room temperature for 5min, monitor by TLC, the reaction of the raw materials is complete, filter with suction, wash with water, After washing with cold anhydrous ether, 0.16 g of a light yellow solid was obtained, with a yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com