Glyantryine family alkaloid derivative, preparation thereof and application of Glyantryine family alkaloid derivative in prevention and treatment of plant viruses, pathogenic bacteria and diseases
A technology of alkaloid derivatives and plant pathogens, applied in the field of agricultural protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
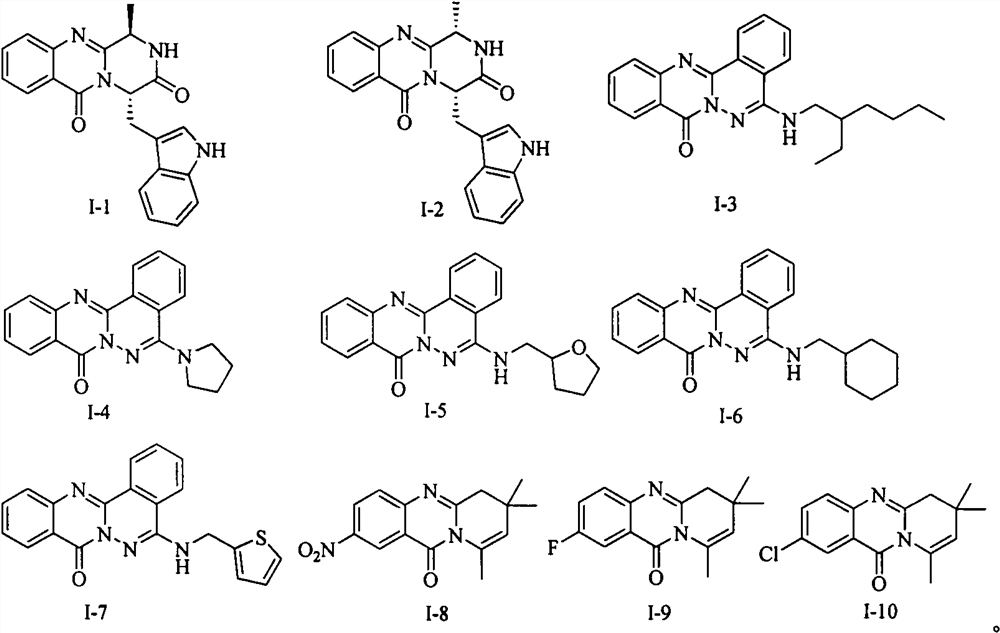
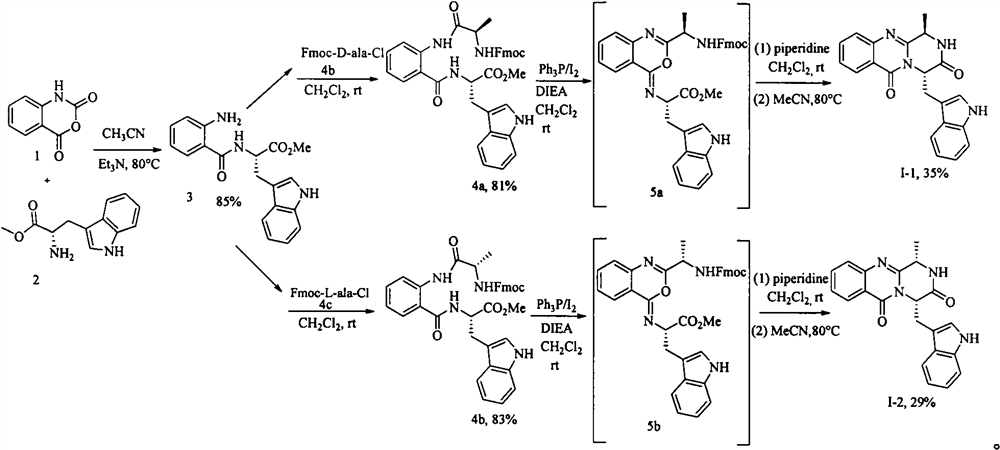
[0015] Example 1: Synthesis of Glyantrypine family alkaloid derivative I-1.
[0016] The first step, the synthesis of intermediate 3. Isatoic anhydride (1) (15 g, 92 mmol) and L-tryptophan methyl ester hydrochloride (2) (24 g, 92 mmol), triethylamine (14 mL, 96.6 mmol) were added to acetonitrile and heated at 80°C. TLC detected the end of the reaction, spin off most of the solvent, add ethyl acetate to dissolve, wash the organic phase with saturated brine, and recrystallize with petroleum ether / ethyl acetate to obtain 26 g of yellow solid, yield 85%, melting point 125-127°C . 1 H NMR (400MHz, CDCl 3 )δ8.21(s, 1H), 7.56(d, J=7.9Hz, 1H), 7.34(d, J=8.1Hz, 1H), 7.20-7.15(m, 3H), 7.10(t, J=7.5 Hz, 1H), 6.99 (d, J=1.9Hz, 1H), 6.69-6.59 (m, 2H), 6.55 (t, J=7.5Hz, 1H), 5.48 (s, 2H), 5.08 (dd, J =12.8, 5.4Hz, 1H), 3.71(s, 3H), 3.42(dd, J=5.2, 1.9Hz, 2H). 13 C NMR (100MHz, CDCl 3 ) δ172.6, 168.8, 148.9, 136.2, 132.6, 127.6, 127.6, 122.9, 122.3, 119.7, 118.7, 117.3, 116.6, 115.3, 1...
Embodiment 2
[0019] Example 2: Synthesis of Glyantrypine family alkaloid derivative I-2.
[0020] The first step, the synthesis of intermediate 4b. Intermediate 3 (5 g, 14.8 mmol) and Fmoc-L-ala-Cl (5.6 g, 17.8 mmol) were added to dichloromethane, and the reaction was carried out at room temperature overnight. The reaction was detected by TLC, and quenched by adding saturated sodium carbonate solution. The organic phase was washed with brine, and recrystallized from dichloromethane / petroleum ether to obtain 6.7 g of a yellow solid with a yield of 83% and a melting point of 128-130°C. 1 H NMR (400MHz, CDCl 3 )δ11.49(s, 1H), 8.58(d, J=8.2Hz, 1H), 8.15(s, 1H), 7.76(d, J=7.2Hz, 2H), 7.66(d, J=6.4Hz, 1H), 7.61-7.55(m, 1H), 7.52-7.44(m, 2H), 7.43-7.37(m, 2H), 7.37-7.27(m, 4H), 7.17(t, J=7.5Hz, 1H) , 7.09-7.03(m, 1H), 6.99(t, J=7.6Hz, 1H), 6.95(s, 1H), 6.73(d, J=7.4Hz, 1H), 5.55(d, J=6.2Hz, 1H), 5.04(dd, J=12.5, 5.2Hz, 1H), 4.50-4.32(m, 3H), 4.30-4.23(m, 1H), 3.73(s, 3H), 3.44-3.31(m, 2H) , ...
Embodiment 3
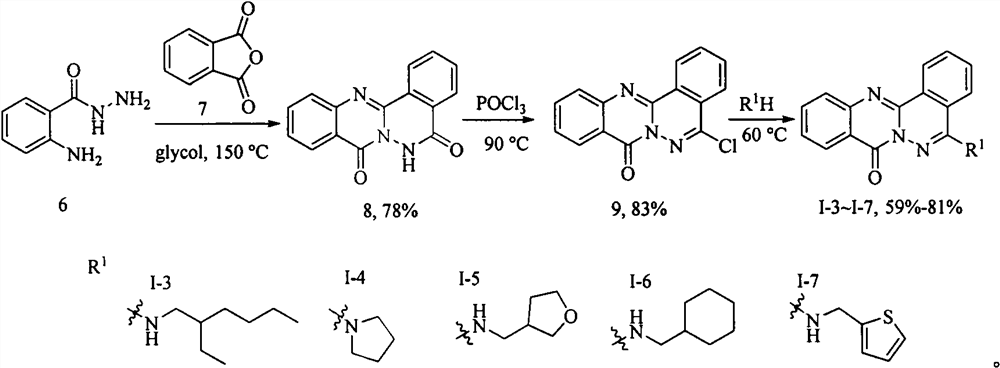
[0022] Example 3: Synthesis of Glyantrypine family alkaloid derivative I-3.
[0023] The first step, the synthesis of intermediate 8. Anthranilic hydrazide (6) (1.51g, 10mmol) and phthalic anhydride (7) (1.48g, 10mmol) were added to ethylene glycol, heated at 150°C, TLC detected the disappearance of the raw materials, cooled to room temperature, and pumped under reduced pressure. After filtering, the filter cake was washed with ethyl acetate to obtain 2.1 g of a khaki solid with a yield of 80% and a melting point of 288-290°C. 1 H NMR (400MHz, DMSO-d 6 )δ8.82(d, J=7.5Hz, 1H), 8.28(dd, J=8.0, 1.0Hz, 1H), 8.20(d, J=7.6Hz, 1H), 8.05-7.93(m, 2H), 7.93-7.89(m, 1H), 7.88-7.84(m, 1H). 7.64-7.56(m, 1H).
[0024] The second step, the synthesis of intermediate 9. Intermediate 8 (1 g, 3.8 mmol) was added to POCl 3 , heated at 90°C, TLC detected the disappearance of the raw materials, cooled to room temperature, slowly poured the reaction solution into ice water, and filtered under r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com