Application of Alkaloid Streptindole and Its Derivatives in Controlling Plant Virus Bacterial Diseases
A technology of derivatives and alkaloids, applied in the field of agricultural protection, can solve the problems of few plant virus inhibitors and few therapeutic agents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
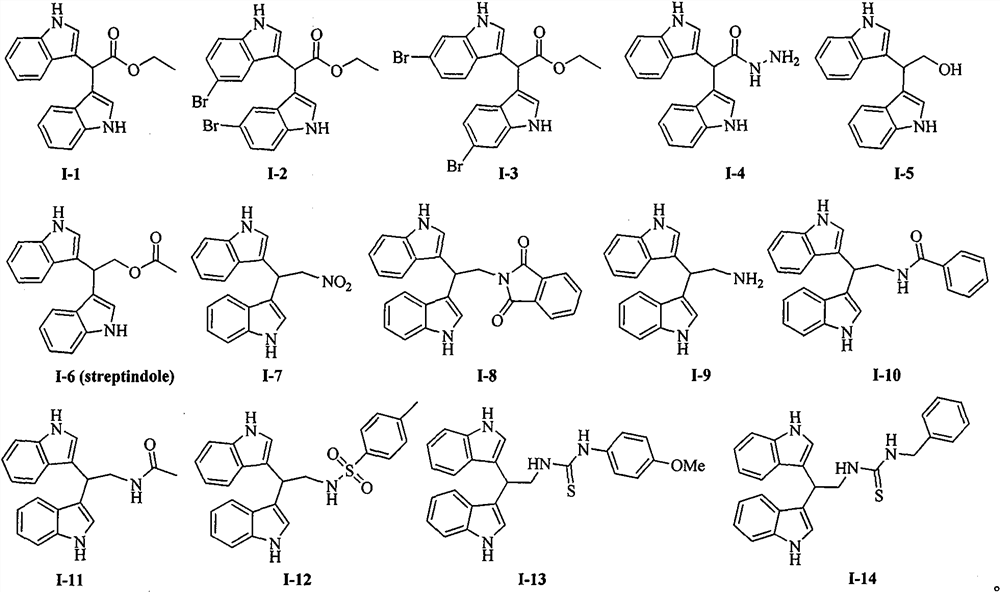
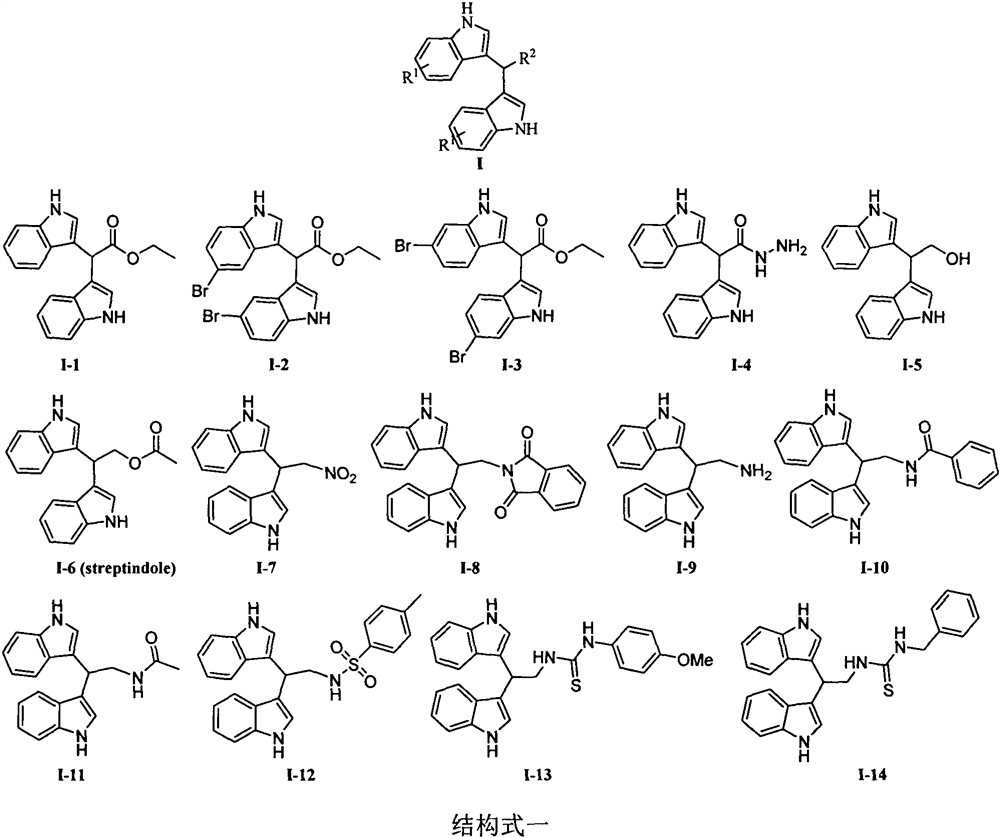
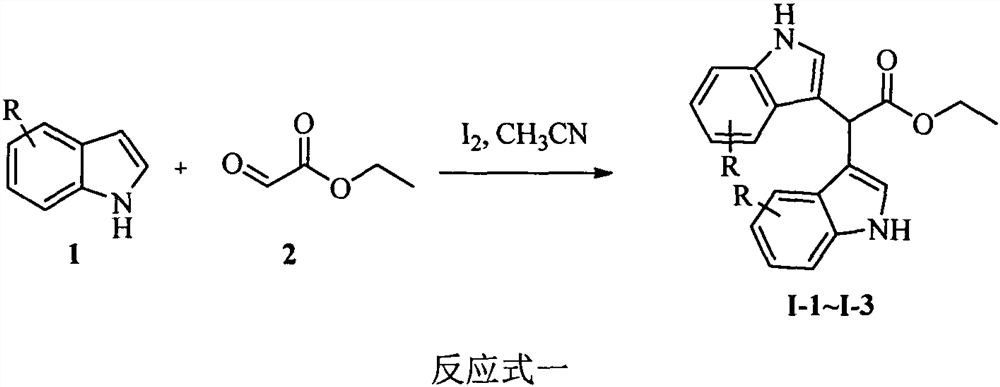
[0022] Example 1: Synthesis of Compounds I-1~I-3: 0°C, under stirring, add the corresponding indole 1 (5mmol) and aldehyde 2 (0.26 g, 2.5mmol) to the acetonitrile solution (25mL) I 2 (0.13 g, 0.5 mmol). Keep stirring at this temperature for 30min, add 5% Na 2 S 2 o 3 solution (20 mL) to quench the reaction. The reaction solution was extracted with ethyl acetate (3×50mL), the organic phase was washed with saturated brine (100mL), anhydrous Na 2 SO 4 Dry, filter and concentrate. The residue was subjected to column chromatography with petroleum ether and ethyl acetate (5:1, v / v) to obtain compounds I-1~I-3.
[0023] Ethyl 2,2-bis(1H-indol-3-yl)acetate (I-1). Brown oil; yield 91%; 1 H NMR (400MHz, CDCl 3 )δ8.03 (s, 2H, NH), 7.67 (d, J=7.9Hz, 2H, Ar-H), 7.30 (t, J=8.1Hz, 2H, Ar-H), 7.23-7.21 (m, 2H, Ar-H), 7.14-7.10(m, 2H, Ar-H), 7.00(d, J=2.0Hz, 2H, Ar-H), 5.53(s, 1H, Ar 2 -CH), 4.25 (q, J = 7.1Hz, 2H, CH 2 ), 1.29(t, J=7.1Hz, 3H, CH 3 ); 13 C NMR (100MHz, CDCl 3 )δ...
Embodiment 2
[0026]Example 2: Synthesis of 2,2-bis(1H-indol-3-yl)acetohydrazide (I-4): under stirring, dissolve ester I-1 (1.59g, 5mmol) in ethanol solution ( 25 mL) was added with 80% hydrazine hydrate (25 mL). Reflux for 3 hours, cool to room temperature, concentrate part of the ethanol, precipitate a large amount of solid, suction filter, wash with water, and dry to obtain white solid compound I-4 (1.26g, 83%), melting point 234-235°C; 1 H NMR (400MHz, DMSO-d 6 ): δ10.87(s, 2H, NH), 9.42(s, 1H, NH 2 -NH), 7.57(d, J=7.9Hz, 2H, Ar-H), 7.34(d, J=8.1Hz, 2H, Ar-H), 7.17(d, J=2.2Hz, 2H, Ar-H ), 7.07-7.03(m, 2H, Ar-H), 6.96-6.92(m, 2H, Ar-H), 5.26(s, 1H, Ar-H 2 -CH), 4.26(s, 2H, NH-NH 2 ); 13 C NMR (100MHz, DMSO-d 6 )δ172.3, 136.6, 127.1, 124.1, 121.3, 119.4, 118.6, 114.3, 111.8, 39.5.
Embodiment 3
[0027] Embodiment 3: Compound 2,2-bis(1H-indol-3-yl)ethanol (I-5) and 2,2-bis(1H-indol-3-yl)ethyl acetate (I- 6) Synthesis of:
[0028] Step 1: Slowly add lithium aluminum tetrahydride (0.57g, 15mmol) to tetrahydrofuran (50mL) under stirring at 0°C, stir for 10min, then slowly add ester I-1 (1.59g, 5mmol), the reaction solution naturally Rise to room temperature, react for 3 h, and slowly drop water (10 mL) into the reaction system to quench the reaction. Add diatomaceous earth for suction filtration, wash the filter cake with ethyl acetate, combine the organic phases with anhydrous Na 2 SO 4 Drying, suction filtration, concentration, and column chromatography gave light yellow oily compound I-5 (1.02 g, 74%); 1 H NMR (400MHz, CDCl 3 )δ7.95(s, 2H, NH), 7.60(d, J=7.9Hz, 2H, Ar-H), 7.17-7.20(m, 4H, Ar-H), 7.09(t, J=6.2Hz, 2H, Ar-H), 6.72(s, 2H, Ar-H), 4.72(t, J=5.9Hz, 1H, Ar 2 -CH), 4.20 (d, J=5.8Hz, 2H, CH 2 ), 2.32(s, 1H, OH); 13 C NMR (100MHz, CDCl 3 )δ136.5, 126.9, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com