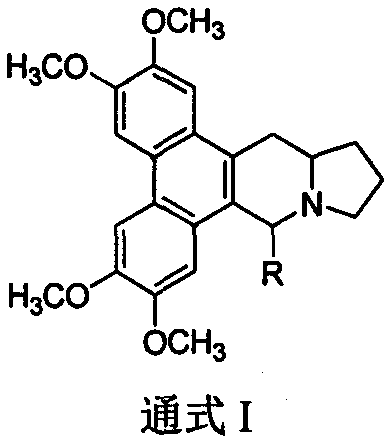
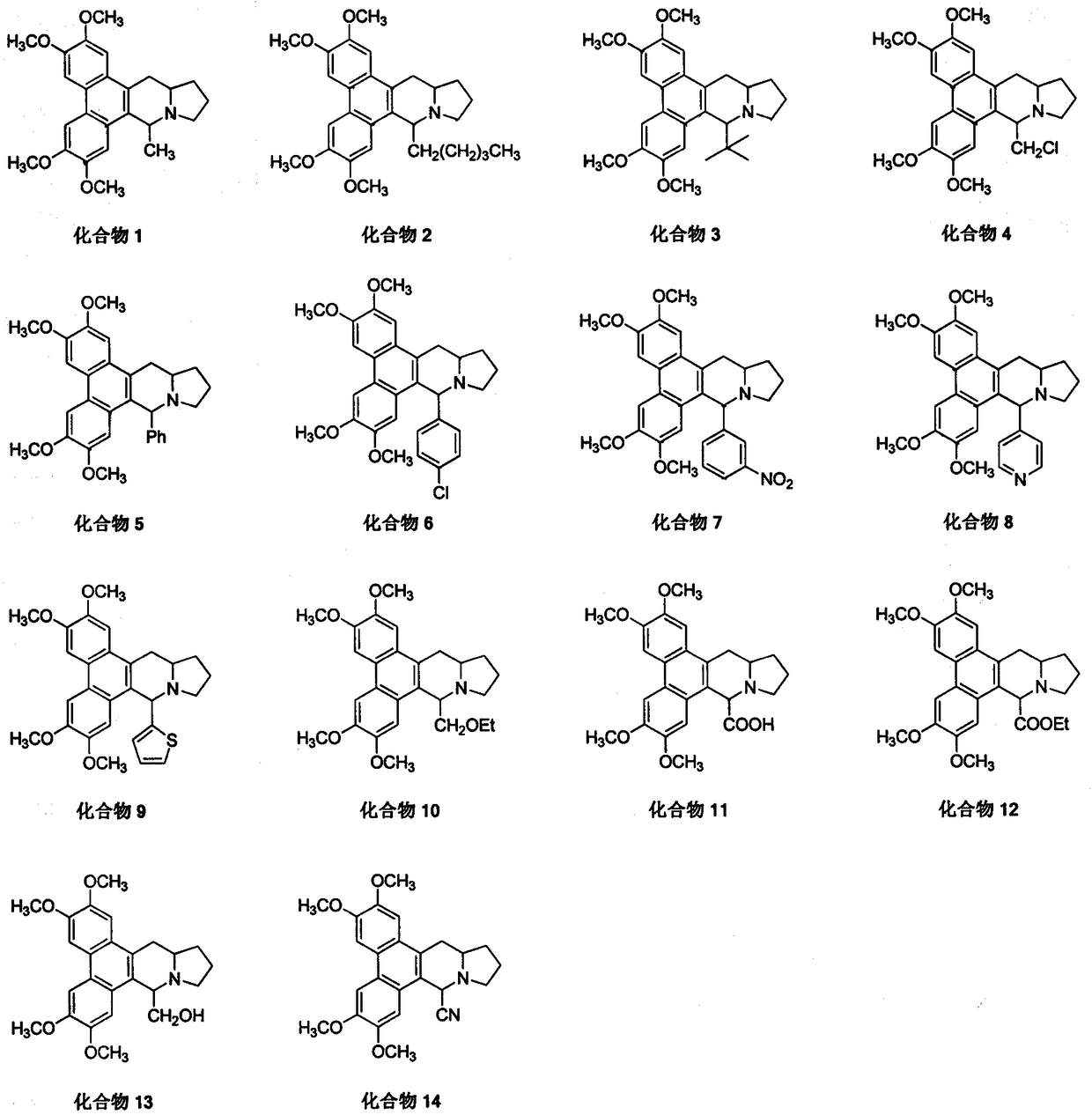
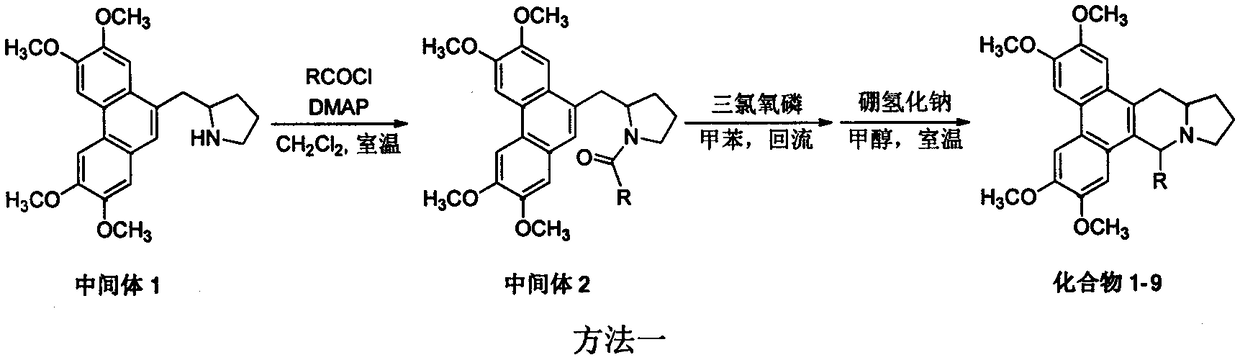
9th-substituted tylophorine derivatives, preparation thereof, and application thereof on inhibiting activity of tobacco mosaic virus
A tobacco mosaic disease, anti-tobacco mosaic technology, applied in the field of 9-substituted sylphenine derivatives and their preparation and application in anti-tobacco mosaic virus activity, can solve the impact of biological activity No literature Reporting, biological activity and other issues, to achieve excellent anti-plant virus activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Synthesis of 9-methylsilomenine (compound 1) (method 1)
[0023]
[0024] Add 0.76g (1.99mmol) of intermediate 1 to a 100mL reaction flask, 30mL of anhydrous dichloromethane, 0.3g (3.82mmol) of acetyl chloride, 0.4g (3.95mmol) of triethylamine, 4-(N,N-di Methylamino)pyridine 0.05g (0.41mmol), stirred at room temperature for 6h, the reaction solution was washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and subjected to normal pressure column chromatography (dichloromethane:methanol=40:1 elution) Obtain white solid 0.42g, productive rate: 49%; The obtained intermediate 2 0.1g (0.24mmol) is joined in the reaction bottle of 100mL, then adds anhydrous toluene 30mL, adds phosphorus oxychloride 1mL (10.92 mmol), heated to reflux for 1 h, removed the solvent by rotary evaporation, added 30 mL of anhydrous methanol, added 0.1 g (2.64 mmol) of sodium borohydride in an ice-water bath, stirred at room temperature for 1 h, a large amo...
Embodiment 2
[0026] The 9-substituted sylphenine derivative (compound 2-9) is completed with reference to the operation steps of Example 1
[0027] Compound 2, white solid, yield 95%; melting point: 164-165°C; 1 H NMR (400MHz, CDCl 3 )δ7.82(s, 1H), 7.81(s, 1H), 7.34(s, 2H), 4.33-4.25(m, 1H), 4.11(s, 6H), 4.06(s, 3H), 4.05(s , 3H), 3.57(t, J=7.2Hz, 1H), 3.25(d, J=14.8Hz, 1H), 2.89-2.76(m, 1H), 2.61-2.51(m, 1H), 2.46(dd, J=16.8, 8.4Hz, 1H), 2.16-1.97(m, 3H), 1.97-1.82(m, 2H), 1.79-1.65(m, 2H), 1.49-1.35(m, 1H), 1.16-0.95( m, 4H), 0.71(t, J=6.8Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ148.7, 148.5, 148.0, 147.9, 130.7, 128.9, 125.8, 124.4, 124.0, 123.3, 105.3, 104.2, 103.5, 103.2, 62.9, 59.0, 56.1, 56.0, 55.9, 53.9, 30.3, 93.3, 34.3 , 24.6, 22.5, 22.0, 14.1; HRMS (ESI) calcd for C 29 h 38 NO 4 + (M+H) + 464.2795, found 464.2804.
[0028] Compound 3, pale yellow solid, yield 18%; melting point: 210-211°C; 1 H NMR (400MHz, CDCl 3 )δ7.81(s, 1H), 7.79(s, 1H), 7.53(s, 1H), 7.35(s, 1H), 4.44...
Embodiment 3
[0035] Example 3 Synthesis of 9-ethoxymethyl sylphenine (compound 10) (method 2)
[0036]
[0037]Add 0.2g (0.45mmol) of compound 4, 20mL of absolute ethanol, 0.05g (0.89mmol) of potassium hydroxide to a 50mL reaction bottle, heat to reflux for 8h, spin dry, add appropriate amount of dichloromethane and water to dissolve, separate liquid, organic phase Washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and precipitated under reduced pressure to obtain 0.12 g of a white solid with a yield of 61% and a melting point of 155-156°C; 1 H NMR (400MHz, CDCl 3 )δ7.81(s, 1H), 7.80(s, 1H), 7.50(s, 1H), 7.32(s, 1H), 4.35-4.29(m, 1H), 4.11(s, 6H), 4.05(s , 6H), 3.97(d, J=9.6Hz, 1H), 3.76(t, J=8.4Hz, 1H), 3.60-3.47(m, 3H), 3.26(d, J=15.2Hz, 1H), 2.94 -2.84(m, 1H), 2.61-2.50(m, 2H), 2.16-1.98(m, 2H), 1.97-1.83(m, 1H), 1.81-1.67(m, 1H), 1.24(t, J= 6.8Hz, 4H); 13 C NMR (100MHz, CDCl 3 )δ 148.82, 148.77, 148.23, 148.18, 129.0, 128.1, 125.8, 124.4, 124.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com