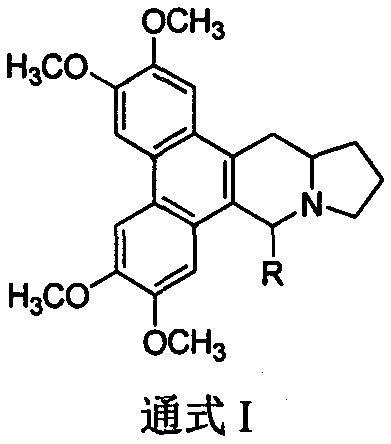
9-substituted seraphine derivatives and their preparation and application in anti-tobacco mosaic virus activity
A technology of tobacco mosaic virus and seraphine, which is applied in the field of 9-substituted seraphine derivatives and their preparation and application in anti-tobacco mosaic virus activity, and can solve the problems of biological activity and biological activity. The impact of no literature reports and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
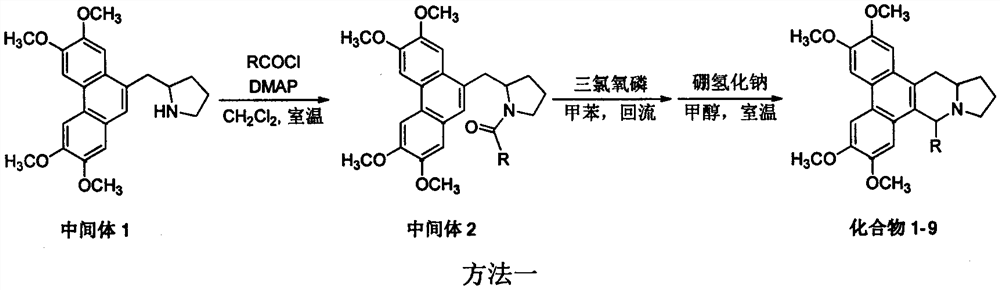
[0022] Example 1 Synthesis of 9-methylsalmenine (compound 1) (method 1)
[0023]
[0024] In a 100 mL reaction flask, 0.76 g (1.99 mmol) of intermediate 1, 30 mL of anhydrous dichloromethane, 0.3 g (3.82 mmol) of acetyl chloride, 0.4 g (3.95 mmol) of triethylamine, 4-(N,N-dichloromethane) were added. Methylamino)pyridine 0.05g (0.41mmol), the reaction was stirred at room temperature for 6h, the reaction solution was washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and subjected to atmospheric pressure column chromatography (elution with dichloromethane:methanol=40:1) 0.42 g of white solid was obtained, yield: 49%; 0.1 g (0.24 mmol) of the obtained intermediate 2 was added to a 100 mL reaction flask, then 30 mL of anhydrous toluene was added, and 1 mL of phosphorus oxychloride (10.92 mL) was added under stirring at room temperature. mmol), heated under reflux for 1 h, removed the solvent by rotary evaporation, added 30 mL of anhydrous meth...
Embodiment 2
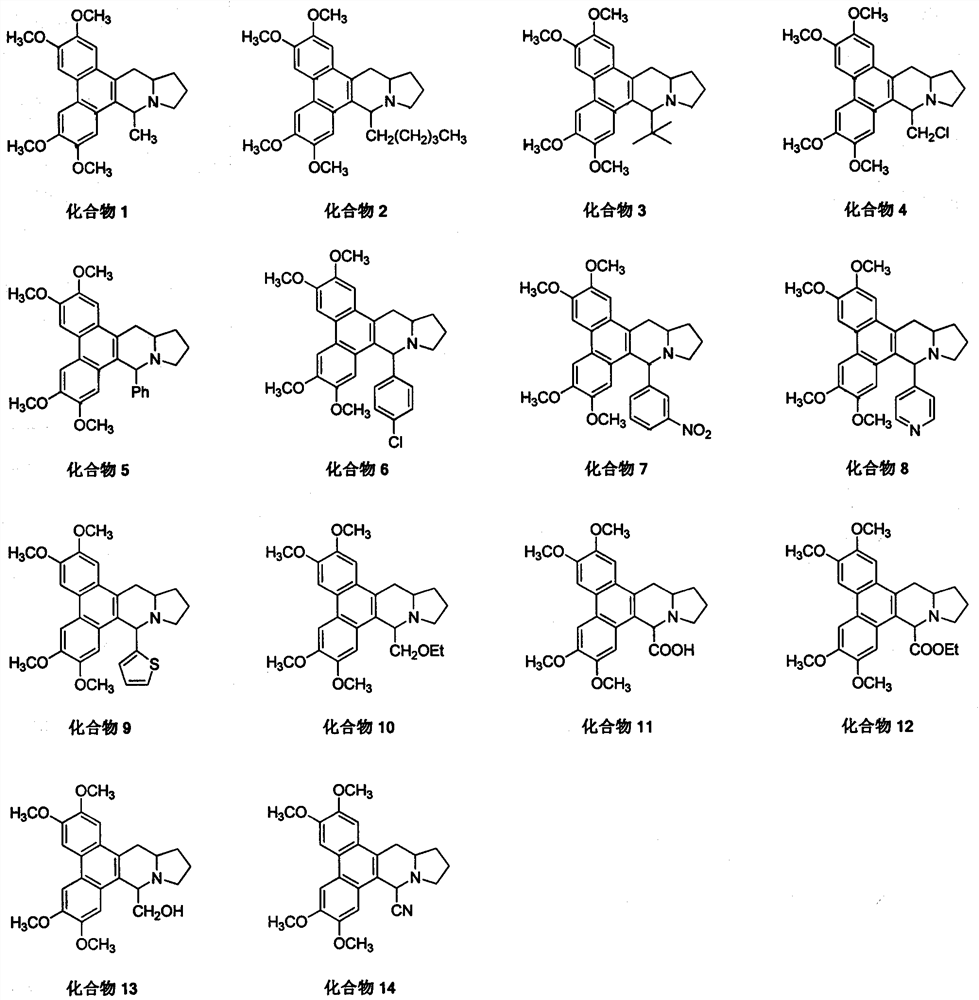
[0026] The 9-substituted salmenine derivatives (compounds 2-9) are completed with reference to the operation steps of Example 1
[0027] Compound 2, white solid, yield 95%; melting point: 164-165°C; 1 H NMR (400MHz, CDCl 3 )δ7.82(s, 1H), 7.81(s, 1H), 7.34(s, 2H), 4.33-4.25(m, 1H), 4.11(s, 6H), 4.06(s, 3H), 4.05(s , 3H), 3.57 (t, J=7.2Hz, 1H), 3.25 (d, J=14.8Hz, 1H), 2.89-2.76 (m, 1H), 2.61-2.51 (m, 1H), 2.46 (dd, J=16.8, 8.4Hz, 1H), 2.16-1.97(m, 3H), 1.97-1.82(m, 2H), 1.79-1.65(m, 2H), 1.49-1.35(m, 1H), 1.16-0.95( m, 4H), 0.71 (t, J=6.8Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ148.7, 148.5, 148.0, 147.9, 130.7, 128.9, 125.8, 124.4, 124.0, 123.3, 105.3, 104.2, 103.5, 103.2, 62.9, 59.0, 56.1, 56.0, 55.9, 53.9, 35.6, 34.3 , 24.6, 22.5, 22.0, 14.1; HRMS(ESI) calcd for C 29 H 38 NO 4 + (M+H) + 464.2795, found 464.2804.
[0028] Compound 3, pale yellow solid, yield 18%; melting point: 210-211°C; 1 H NMR (400MHz, CDCl 3 )δ7.81(s, 1H), 7.79(s, 1H), 7.53(s, 1H), 7.35(s, 1H), 4.4...
Embodiment 3
[0035] Example 3 Synthesis of 9-ethoxymethyl salmenine (compound 10) (method 2)
[0036]
[0037]Add 0.2 g (0.45 mmol) of compound 4, 20 mL of absolute ethanol, 0.05 g (0.89 mmol) of potassium hydroxide to a 50 mL reaction flask, heat under reflux for 8 h, spin dry, add an appropriate amount of dichloromethane and water to dissolve, separate liquids, and the organic phase Washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and desolvated under reduced pressure to obtain 0.12 g of white solid, yield 61%, melting point 155-156°C; 1 H NMR (400MHz, CDCl 3 )δ7.81(s, 1H), 7.80(s, 1H), 7.50(s, 1H), 7.32(s, 1H), 4.35-4.29(m, 1H), 4.11(s, 6H), 4.05(s , 6H), 3.97(d, J=9.6Hz, 1H), 3.76(t, J=8.4Hz, 1H), 3.60-3.47(m, 3H), 3.26(d, J=15.2Hz, 1H), 2.94 -2.84(m, 1H), 2.61-2.50(m, 2H), 2.16-1.98(m, 2H), 1.97-1.83(m, 1H), 1.81-1.67(m, 1H), 1.24(t, J= 6.8Hz, 4H); 13 C NMR (100MHz, CDCl 3 )δ148.82, 148.77, 148.23, 148.18, 129.0, 128.1, 125.8, 124.4, 124.0, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com