Application of dehydrogenized derivatives of tylophorine in inhibiting activity of tobacco mosaic virus
A tobacco mosaic disease, anti-tobacco mosaic technology, applied in the application field of seraphine dehydrogenation derivatives in anti-tobacco mosaic virus activity, can solve the problems of high toxicity, poor water solubility, and impact on the central nervous system, Achieve excellent anti-plant virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19
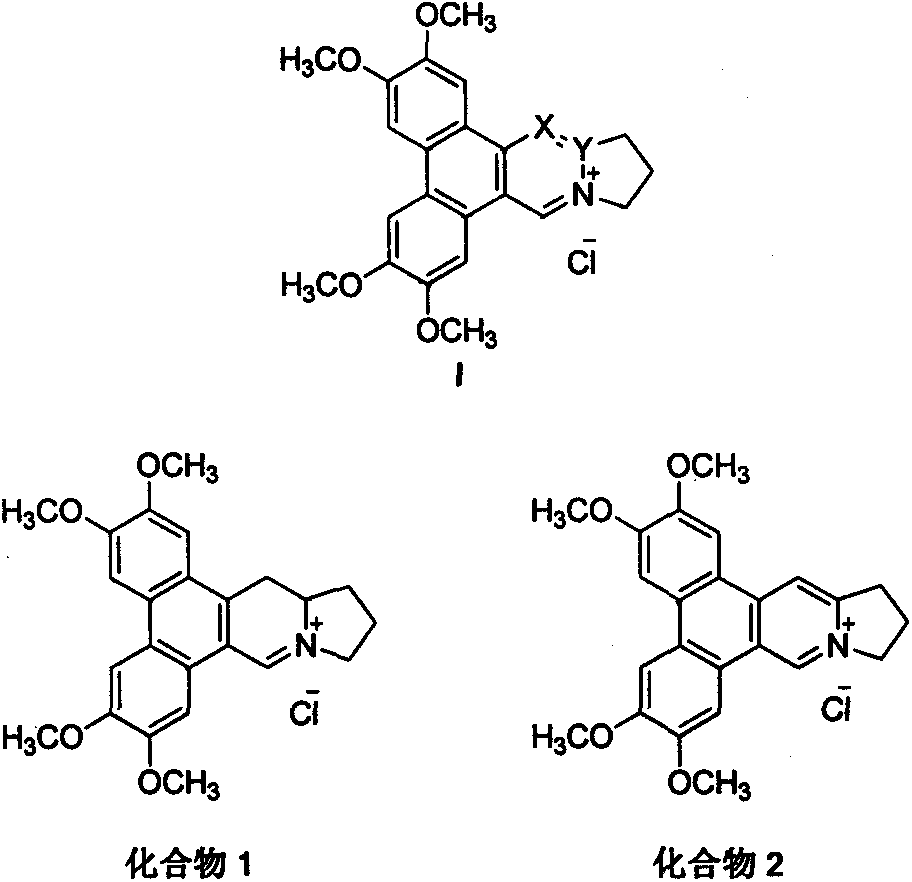
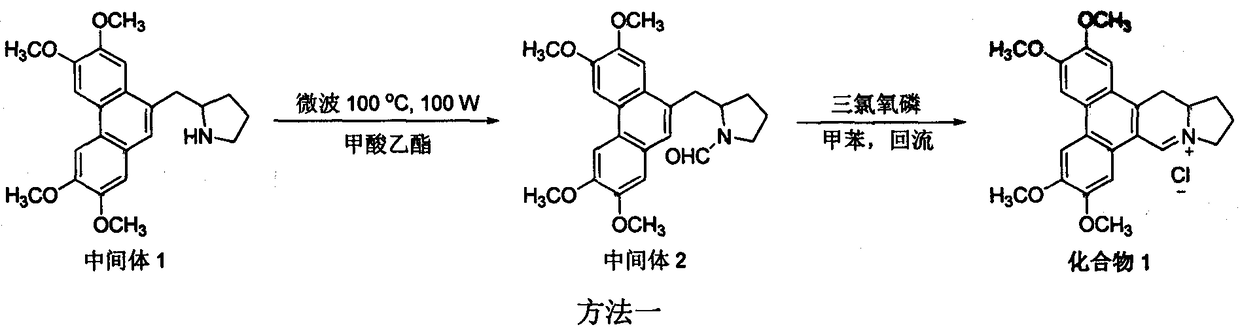
[0012] Example 1 Synthesis of 9-dehydrosylmenine hydrochloride (compound 1) (method 1)
[0013]
[0014] Add 0.2g (0.52mmol) of Intermediate 1 and 20mL of ethyl formate to a 35mL microwave reactor, set T=100°C, P=100W, P max =250psi, react for 1h. Desolvation under reduced pressure, dichloromethane dilution, 3mol / L hydrochloric acid washing, water washing, saturated brine washing, anhydrous sodium sulfate drying, normal pressure column chromatography (dichloromethane:methanol=40:1), precipitation to obtain intermediate Body 2 white solid 0.2g, yield 95%.
[0015] Add 0.2g (0.49mmol) of intermediate 2 and 20mL of anhydrous toluene to a 50mL reaction flask, add 0.75g (4.89mmol) of phosphorus oxychloride under stirring at room temperature, heat and reflux for 1h, filter with suction, and wash the filter cake with a small amount of methanol , to obtain 0.2 g of orange-yellow solid, yield 95%, melting point: 255-256 ° C; 1 H NMR (300MHz, CDCl 3 )δ11.07(s, 1H), 8.05(s, 1H), 7...
Embodiment 2
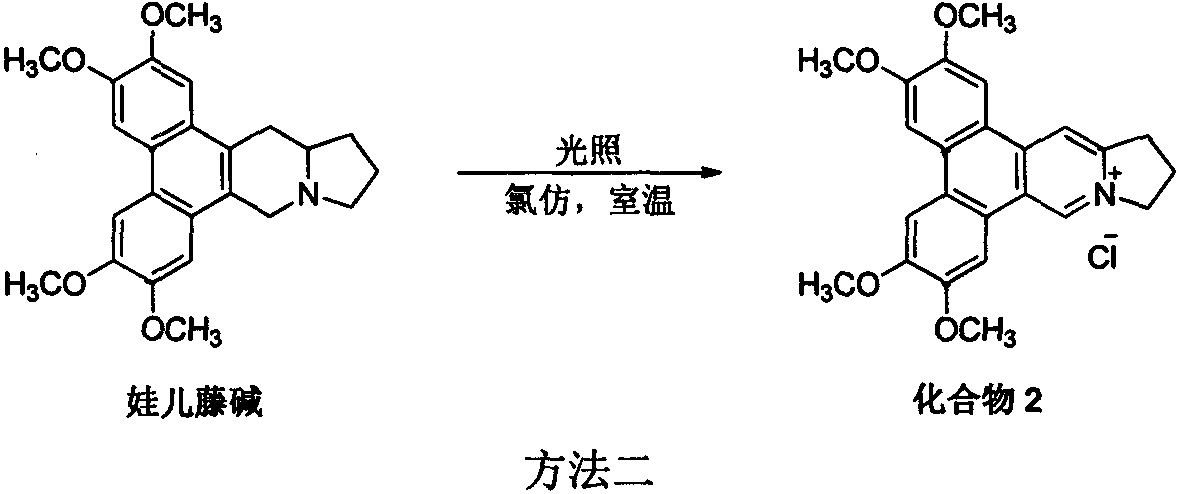
[0016] Example 2 Synthesis of 9,13a,14-dehydrosilomenine hydrochloride (compound 2) (method 2)
[0017]
[0018] Add 39.3 mg (0.1 mmol) of sylphenamine and 50 mL of chloroform into a clean and transparent 100 mL round bottom flask, and replace the air in the system with argon three times. The reaction was stirred at room temperature under direct sunlight until conversion was complete. After desolvation under reduced pressure, normal-pressure column chromatography was performed on a basic alumina column (dichloromethane:methanol=40:1 elution) to obtain a yellow-green solid. 1 HNMR (400MHz, DMSO-d 6 )δ10.42(s, 1H), 9.05(s, 1H), 8.00(s, 2H), 7.59(s, 2H), 5.02-4.87(m, 2H), 4.12-3.98(m, 12H), 3.58 -3.48(m, 3H), 2.63-2.53(m, 2H). 13 C NMR (100MHz, DMSO) δ152.7, 150.6, 149.8, 149.4, 149.1, 138.2, 137.9, 127.5, 123.9, 123.2, 119.3, 118.5, 116.2, 106.1, 104.6, 104.4, 104.3, 57.9, 56.5, 5 , 55.9, 30.9, 21.9. HRMS (ESI) calcd for C 24 h 24 NO 4 (M-Cl) + 390.1700, found 390.170...
Embodiment 3
[0019] Embodiment 3: Conventional in vivo bioassay measures the activity of anti-tobacco mosaic virus
[0020] 1. Virus purification and concentration determination:
[0021] Virus purification and concentration determination were carried out in accordance with the SOP specification for tobacco mosaic virus compiled by the Laboratory of Bioassay, Institute of Elements, Nankai University. After the crude virus extract was centrifuged twice with polyethylene glycol, the concentration was measured and refrigerated at 4°C for later use.
[0022] 2. Compound solution preparation:
[0023] After weighing, the original drug was dissolved in DMF to prepare 1×10 5 μg / mL stock solution, and then diluted to the required concentration with aqueous solution containing 1‰ Tween 80.
[0024] 3. In vivo protection:
[0025] Select Shanxi tobacco with uniform growth at the 3-5 leaf stage, spray the whole plant, and repeat each treatment 3 times, and set a 1‰ Tween 80 aqueous solution as a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com