Application of ellagic acid to preparation of medicine for preventing and treating multiple sclerosis
A technology for multiple sclerosis and ellagic acid, which is applied in the fields of biomedicine and pharmaceutical applications, and can solve the problems of limited drugs and no reported effects in the literature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
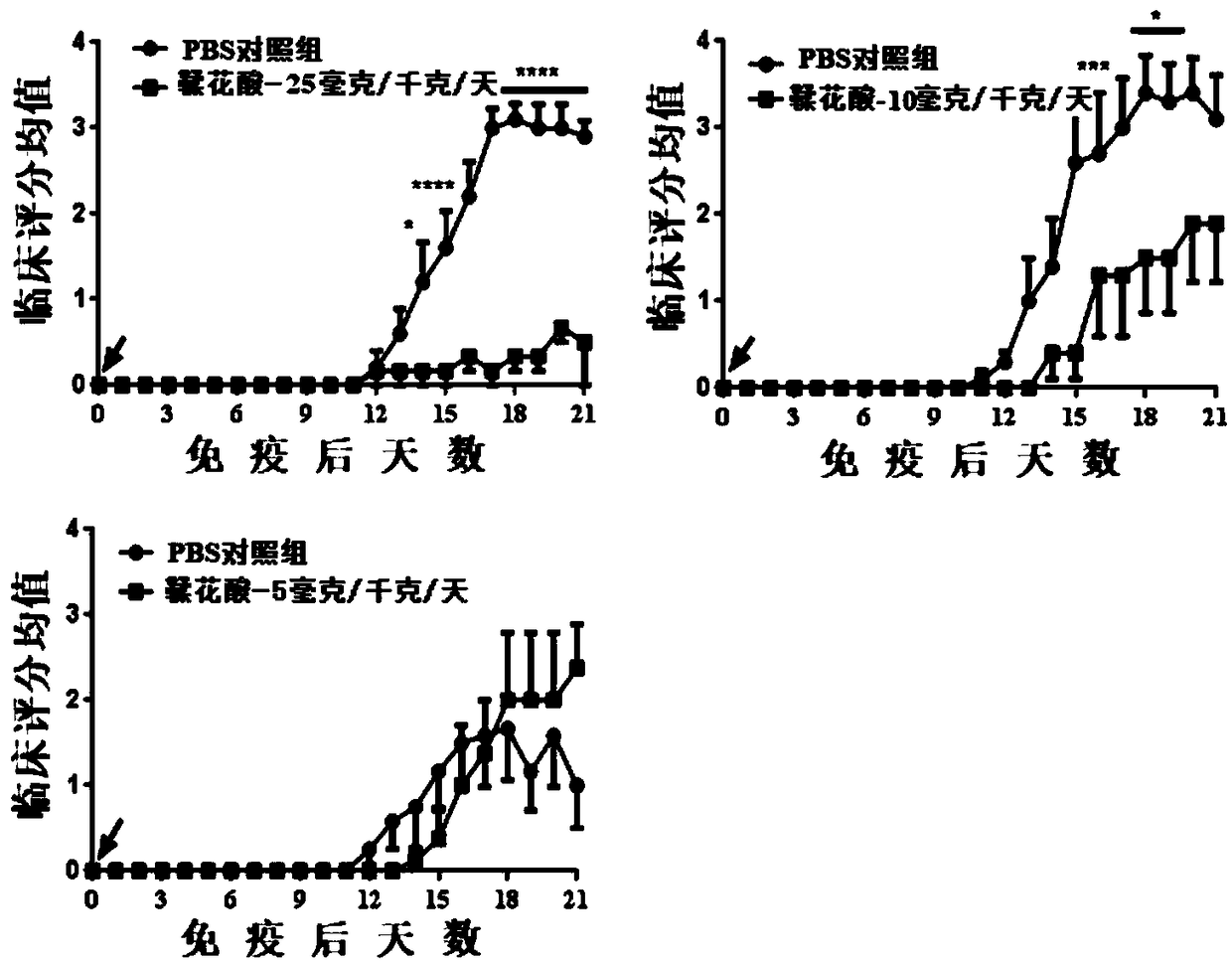
[0022] Different doses of ellagic acid used to improve acute-experimental autoimmune encephalomyelitis
[0023] 1. Experimental treatment
[0024] (1) Establish EAE model
[0025] Eight-week-old female C57BL / 6J mice were purchased from Sibeifu (Beijing) Biotechnology Co., Ltd. Myelin oligodendrocyte glycoprotein peptide (MOG 35-55 ), purchased from Genescript Company, pertussis toxin, purchased from Sigma-Aldrich Company, complete Freund's adjuvant containing Mycobacterium tuberculosis, purchased from BD Difco Company.
[0026] 200 μL containing 200 μg myelin oligodendrocyte glycoprotein peptide (MOG 35-55 ), 50% volume of complete Freund's adjuvant and 5 mg / mL Mycobacterium tuberculosis H37Ra (Sigma-Aldrich) mixed into an emulsion and subcutaneously immunized mice at two sites on the back. Pertussis toxin dilution (200ng / cause) was injected intraperitoneally on the day of immunization and 2 days later.
[0027] Grouping and dosing:
[0028] ①PBS control group: PBS was a...
Embodiment 2
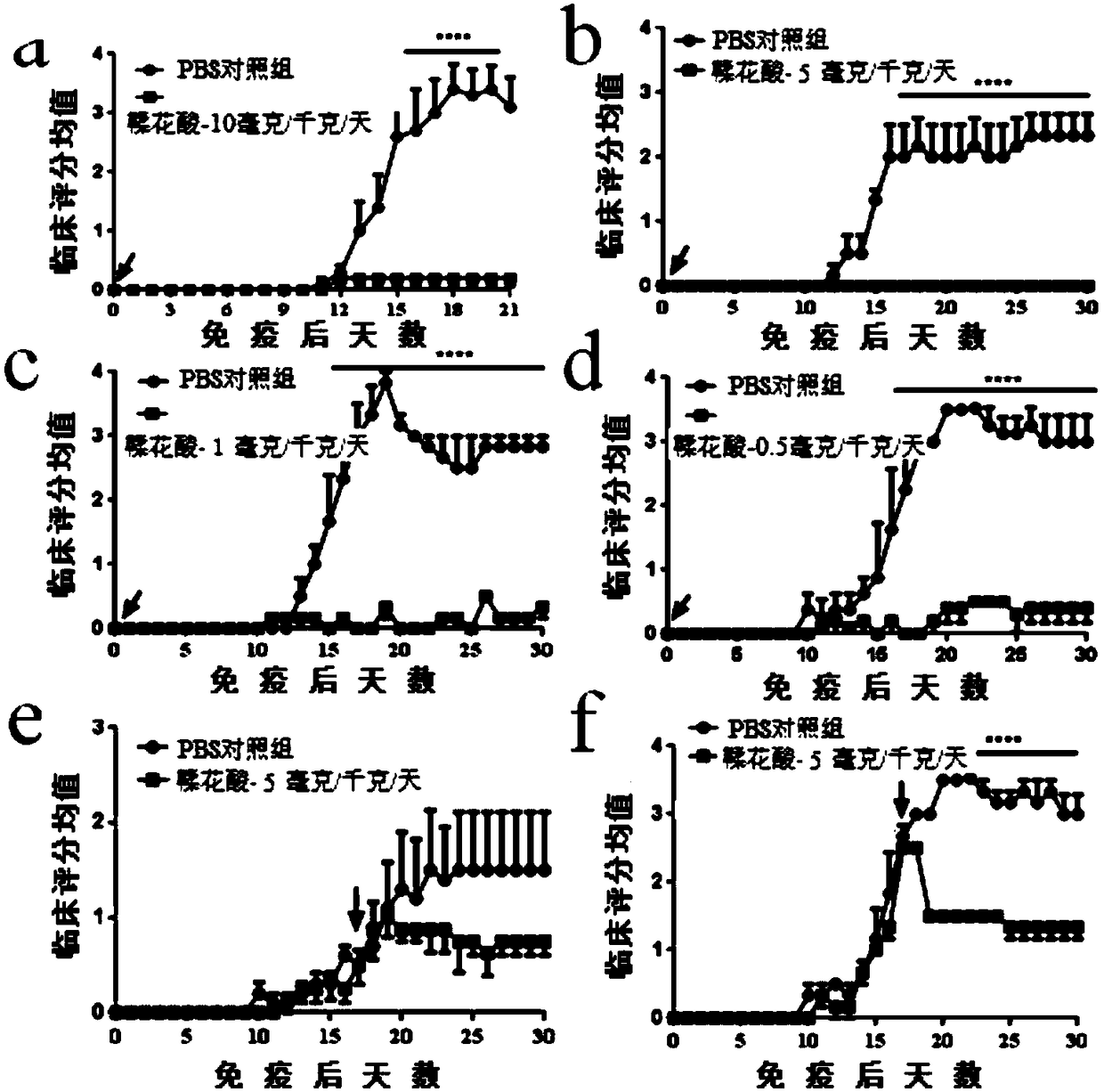
[0035] Different doses of intraperitoneal injection and different treatment stages of ellagic acid for the improvement of acute-experimental autoimmune encephalomyelitis experiment
[0036] 1. Experimental treatment
[0037] The EAE model was established according to the method of Example 1.
[0038] Grouping and dosing:
[0039] ①PBS control group: Intraperitoneal injection was performed every day from the day of immunization.
[0040] ②Ellagic acid treatment group: On the day of immunization, different doses of ellagic acid (10 mg / kg, 5 mg / kg, 1 mg / kg, 0.5 mg / kg) were injected into the intraperitoneal cavity every day, and in the early stage and high incidence stage of the disease, respectively, every day Ellagic acid (5 mg / kg) was administered by intraperitoneal injection.
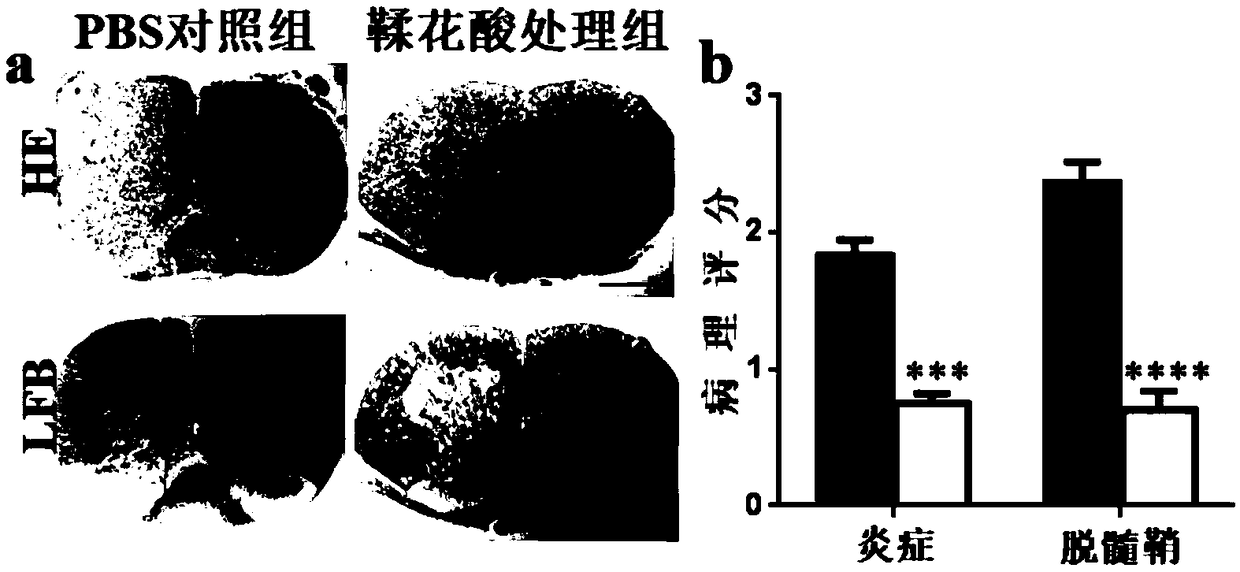
[0041] 2. Post-experiment processing
[0042] (1) EAE clinical score: the same scoring standard as in Example 1, the clinical score was performed on the onset of the mice every day, and the neurolog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com