Synthesis method of key sofosbuvir intermediate
An intermediate, red aluminum technology, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of many reaction by-products, poor trifluoroethanol selectivity, inconvenient reaction and the like, and achieves the effects of low price, good selectivity and production cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
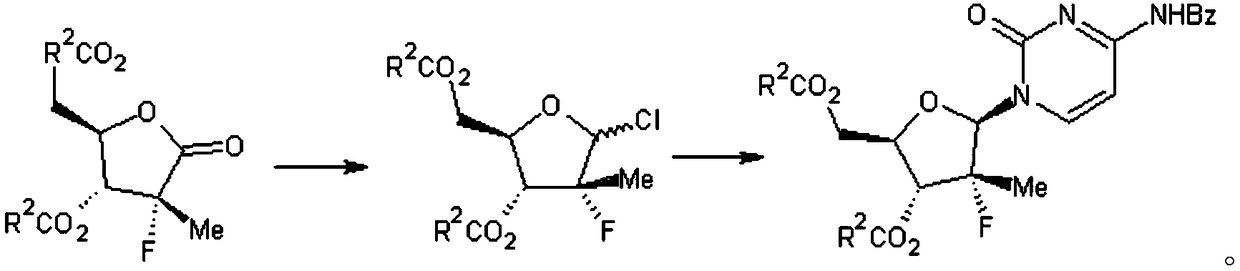
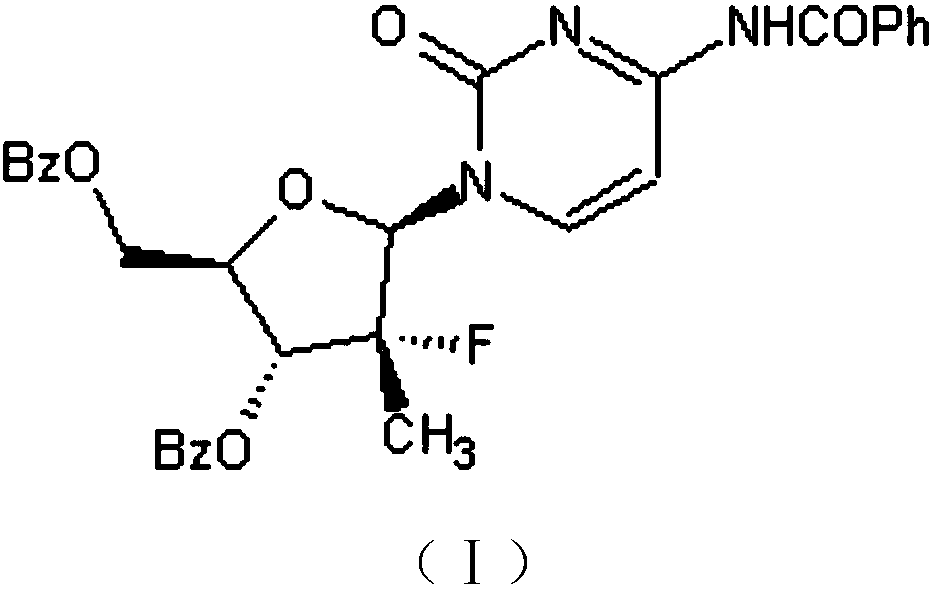
[0024] A kind of preparation method of sofosbuvir intermediate (I), concrete steps are as follows:
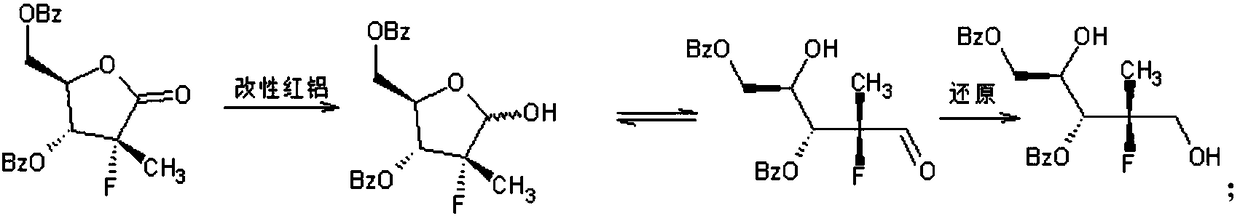
[0025] Step 1, preparation of compound (Ⅲ): In a dry three-necked flask, weigh 61g (0.21mol) of 70% red aluminum and 15g of toluene, then cool the mixture to -15°C, and then drop it into the three-necked flask The mixed solution of 16.5g (0.19mol) of morphine and 10g of toluene, during this process, the temperature is controlled at -10°C, after dropping, keep warm for 30min, slowly rise to 25±5°C, add dropwise to the end of the temperature rise, and obtain the modified red aluminum solution spare.
[0026] Put 49.4g (0.133mol) of compound (II) and 150g of toluene into a dry three-necked flask, cool down to -15°C, add the modified red aluminum solution prepared above dropwise, raise the temperature to 15°C after dropping, and keep it warm for 24h After that, the starting material disappears, and the compound (Ⅲ) is obtained.
[0027] Step 2, preparation of compound (Ⅳ): on the...
Embodiment 2
[0031] A kind of preparation method of sofosbuvir intermediate (I), concrete steps are as follows:
[0032] Step 1, preparation of compound (III): In a dry three-necked flask, weigh 70.7g (0.21mol) of 60% red aluminum and 15g of toluene, then cool the mixture to -20°C, and then drop into the three-necked flask The mixed solution of 21.4g (0.25mol) of morpholine and 10g of toluene, during this process, the temperature is controlled at -15°C, after dropping, keep warm for 20min, slowly rise to 25±5°C, add dropwise to the end of the temperature rise, to obtain modified red aluminum The solution is ready for use.
[0033] Throw 52.4g (0.141mol) of compound (II) and 150g of toluene into a dry three-necked flask, cool down to -15°C, add the modified red aluminum solution prepared above dropwise, raise the temperature to 15°C after dropping, and keep it warm for 20 hours After that, the starting material disappears, and the compound (Ⅲ) is obtained.
[0034]Step 2, preparation of c...
Embodiment 3
[0038] A kind of preparation method of sofosbuvir intermediate (I), concrete steps are as follows:
[0039] Step 1, preparation of compound (Ⅲ): In a dry three-necked flask, weigh 68.9g (0.22mol) of 65% red aluminum and 15g of toluene, then cool the mixture to -15°C, and then drop into the three-necked flask The mixed solution of 14g (0.16mol) of morpholine and 10g of toluene, during this process, the temperature is controlled at -20°C, after dropping, keep warm for 40°C, slowly rise to 25±5°C, add dropwise to the end of the temperature rise, and obtain the modified red aluminum solution spare.
[0040] Feed 53.8g (0.145mol) of compound (II) and 150g of toluene on the spot plate, cool down to -15°C, add the modified red aluminum solution prepared above dropwise, raise the temperature to 25°C and keep it warm after dropping, and react for 26 hours Until the spot plate raw material disappears, compound (Ⅲ) is obtained.
[0041] Step 2, the preparation of compound (Ⅳ): on the b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com