Preparation method of carbamazepine solid dispersion with high drug loading capacity
A solid dispersion, carbamazepine technology, applied in pharmaceutical formulations, medical preparations with non-active ingredients, drug combinations, etc., can solve the problems of limited application, small drug loading, etc., achieve fast dissolution rate, good tableting performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
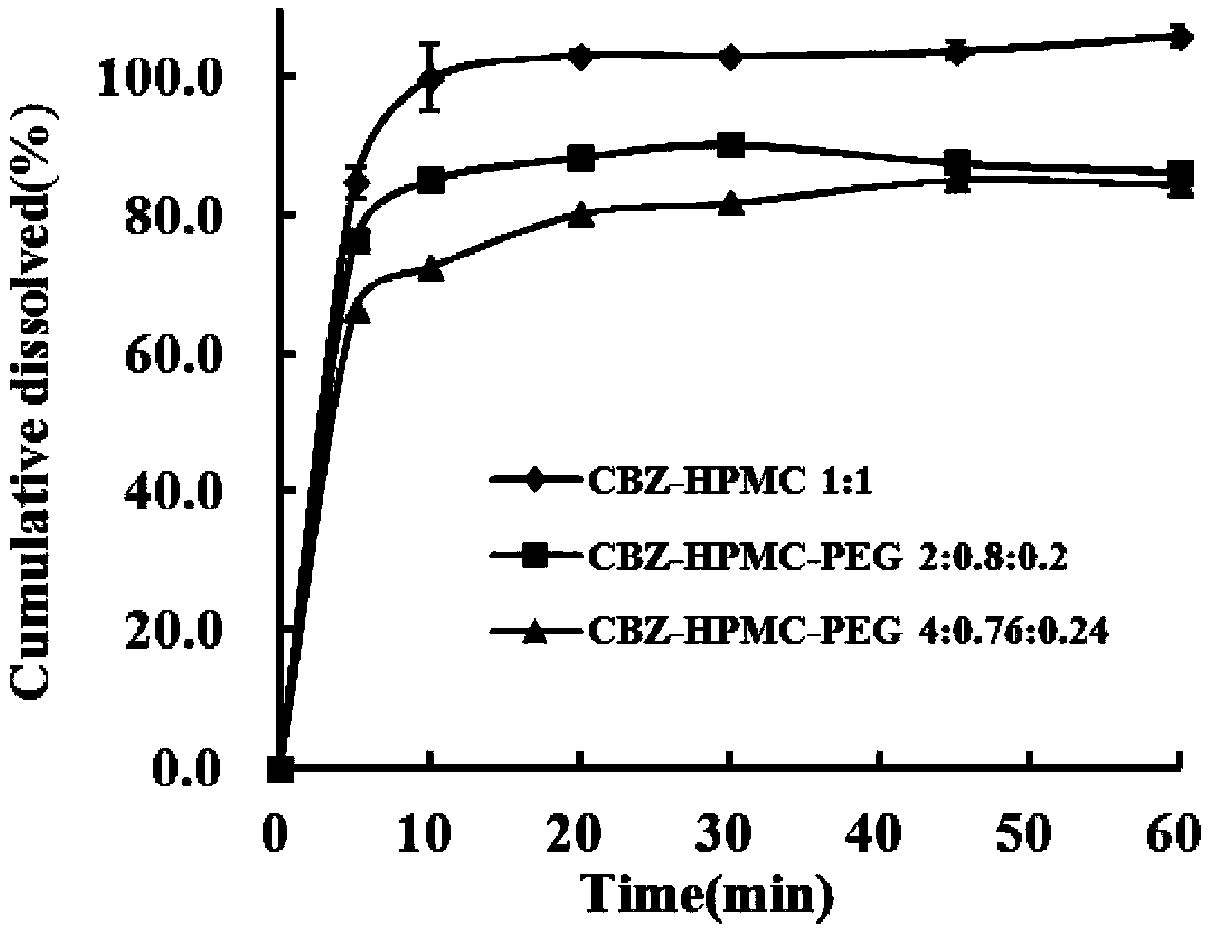
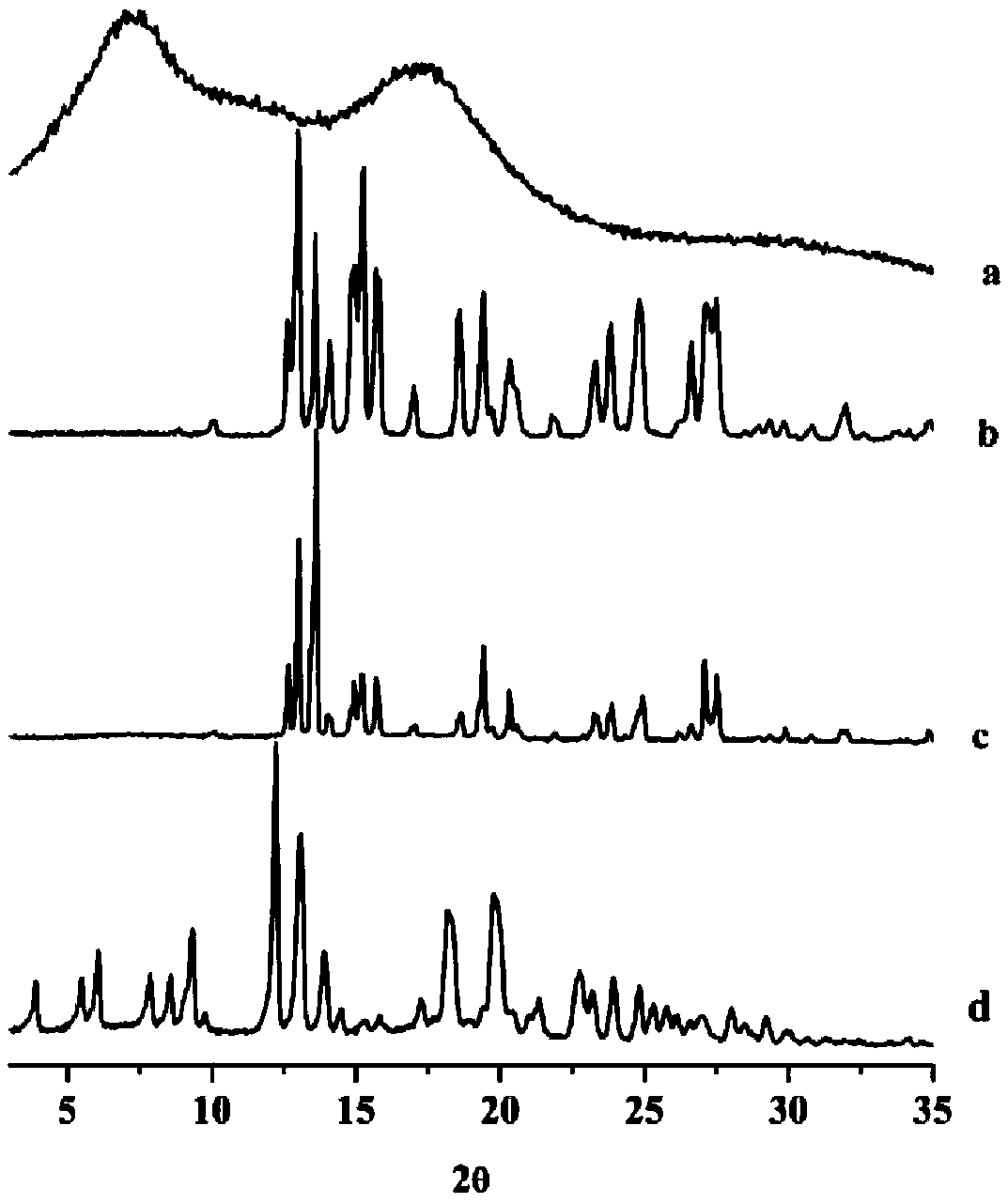
Image
Examples
Embodiment 1
[0023] Take by weighing carbamazepine (Zhejiang Jiuzhou Pharmaceutical Co., Ltd.) 8g, polyacrylic resin Eudragit EPO (Evonik Industrial Group) 8g, mix and add to twin-screw hot-melt extruder (Thermo Fisher Scientific Co., Ltd. company), extruded at 120°C at a speed of 50 rpm, cooled, crushed, and passed through a 120-mesh sieve.
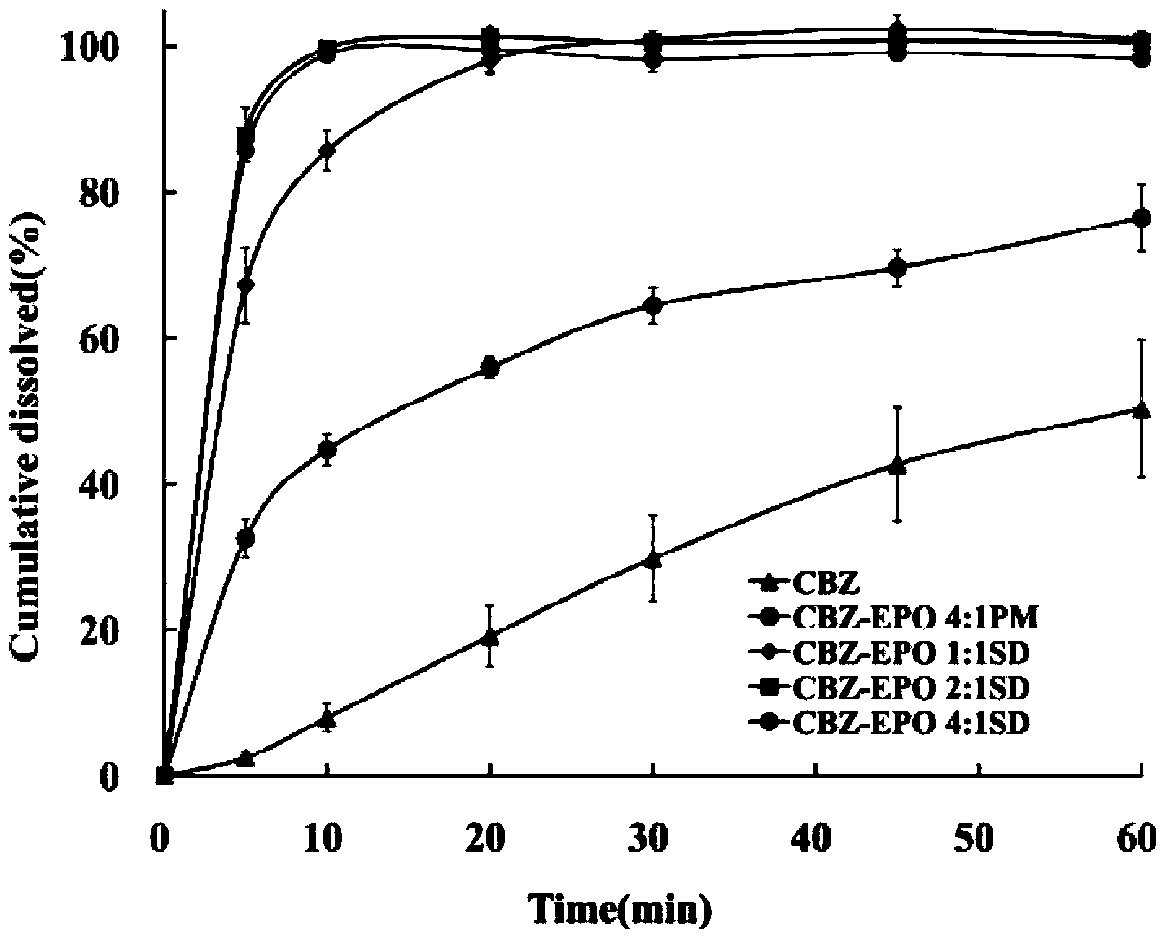
[0024] Get 200mg of gained solid dispersion powder, measure the dissolution rate of medicine by " Chinese Pharmacopoeia " dissolution rate second method, dissolution medium is the hydrochloric acid solution that 500mL pH is 1.2, and rotating speed is 50r min -1 , The sampling time is 5, 10, 20, 30, 45, 60 minutes respectively. As a result, the dissolution in 10min exceeds 80% (see figure 1 ).
Embodiment 2
[0026] Weigh 10g of carbamazepine and 5g of polyacrylic acid resin Eudragit EPO, mix them evenly, add them to a twin-screw hot-melt extruder, extrude them at a speed of 50 rpm at 140°C, crush them after cooling, and pass through a 120-mesh sieve , that is.
[0027] Get product 150mg, according to " Chinese Pharmacopoeia " dissolution rate second method is measured the dissolution rate of medicine, dissolution medium is the hydrochloric acid solution that 500mLpH is 1.2, and rotating speed is 50r min -1 , The sampling time is 5, 10, 20, 30, 45, 60 minutes respectively. As a result, the dissolution in 10min exceeds 80% (see figure 1 ).
Embodiment 3
[0029] Weigh 12g of carbamazepine and 3g of polyacrylic acid resin Eudragit EPO, mix them evenly, add them to a twin-screw hot-melt extruder, extrude them at a speed of 30 rpm at 140°C, crush them after cooling, and pass through a 120-mesh sieve , that is.
[0030] Get product 125mg, measure the dissolution rate of medicine by " Chinese Pharmacopoeia " dissolution rate second method, dissolution medium is the hydrochloric acid solution that 500mLpH is 1.2, and rotating speed is 50r min -1 , The sampling time is 5, 10, 20, 30, 45, 60 minutes respectively. In addition, 100 mg of carbamazepine raw material of 120 mesh and 125 mg of a physical mixture of carbamazepine and acrylic resin with a mass ratio of 4:1 were taken, and the dissolution rate was determined by the same method.
[0031] Result: the dissolution rate of carbamazepine only dissolved 50% in 60min, after adding the polyacrylic acid resin Eudragit EPO of 1 / 4 of drug quality and mixing, the dissolution rate in 60min ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com